Retrograde facilitation of efferent synapses on cochlear hair cells
- PMID: 23183877
- PMCID: PMC3540278
- DOI: 10.1007/s10162-012-0361-0
Retrograde facilitation of efferent synapses on cochlear hair cells
Abstract
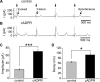
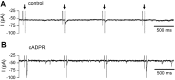
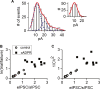
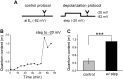
Cochlear inner hair cells (IHCs) are temporarily innervated by efferent cholinergic fibers prior to the onset of hearing. During low-frequency firing, these efferent synapses have a relatively low probability of transmitter release but facilitate strongly with repetitive stimulation. A retrograde signal from the hair cell to the efferent terminal contributes to this facilitation. When IHCs were treated with the ryanodine receptor agonist, cyclic adenosine phosphoribose (cADPR), release probability of the efferent terminal rose. This effect was quantified by computing the quantum content from a train of 100 suprathreshold stimuli to the efferent fibers. Quantum content was sevenfold higher when IHCs were treated with 100 μM cADPR (applied in the recording pipette). Since cADPR is membrane impermeant, this result implies that an extracellular messenger travels from the hair cell to the efferent terminal. cADPR is presumed to generate this messenger by increasing cytoplasmic calcium. Consistent with this presumption, voltage-gated calcium flux into the IHC also caused retrograde facilitation of efferent transmission. Retrograde facilitation was observed in IHCs of a vesicular glutamate transporter (VGlut3) null mouse and for wild-type rat hair cells subject to wide-spectrum glutamate receptor blockade, demonstrating that glutamate was unlikely to be the extracellular messenger. Rather, bath application of nitric oxide (NO) donors caused an increase in potassium-evoked efferent transmitter release while the NO scavenger carboxy-PTIO was able to prevent retrograde facilitation produced by cADPR or IHC depolarization. Thus, hair cell activity can drive retrograde facilitation of efferent input via calcium-dependent production of NO.
Figures





Similar articles
-
Short-term plasticity and modulation of synaptic transmission at mammalian inhibitory cholinergic olivocochlear synapses.Front Syst Neurosci. 2014 Dec 2;8:224. doi: 10.3389/fnsys.2014.00224. eCollection 2014. Front Syst Neurosci. 2014. PMID: 25520631 Free PMC article. Review.
-
Efferent Inhibition of the Cochlea.Cold Spring Harb Perspect Med. 2019 May 1;9(5):a033530. doi: 10.1101/cshperspect.a033530. Cold Spring Harb Perspect Med. 2019. PMID: 30082454 Free PMC article. Review.
-
Facilitating efferent inhibition of inner hair cells in the cochlea of the neonatal rat.J Physiol. 2005 Jul 1;566(Pt 1):49-59. doi: 10.1113/jphysiol.2005.087460. Epub 2005 May 5. J Physiol. 2005. PMID: 15878942 Free PMC article.
-
Ca(2+) and Ca(2+)-activated K(+) channels that support and modulate transmitter release at the olivocochlear efferent-inner hair cell synapse.J Neurosci. 2010 Sep 8;30(36):12157-67. doi: 10.1523/JNEUROSCI.2541-10.2010. J Neurosci. 2010. PMID: 20826678 Free PMC article.
-
Developmental Synaptic Changes at the Transient Olivocochlear-Inner Hair Cell Synapse.J Neurosci. 2019 May 1;39(18):3360-3375. doi: 10.1523/JNEUROSCI.2746-18.2019. Epub 2019 Feb 12. J Neurosci. 2019. PMID: 30755493 Free PMC article.
Cited by
-
Co-release of GABA and ACh from medial olivocochlear neurons fine tunes cochlear efferent inhibition.bioRxiv [Preprint]. 2024 Aug 16:2024.08.12.607644. doi: 10.1101/2024.08.12.607644. bioRxiv. 2024. PMID: 39185230 Free PMC article. Preprint.
-
Short-term plasticity and modulation of synaptic transmission at mammalian inhibitory cholinergic olivocochlear synapses.Front Syst Neurosci. 2014 Dec 2;8:224. doi: 10.3389/fnsys.2014.00224. eCollection 2014. Front Syst Neurosci. 2014. PMID: 25520631 Free PMC article. Review.
-
Efferent Inhibition of the Cochlea.Cold Spring Harb Perspect Med. 2019 May 1;9(5):a033530. doi: 10.1101/cshperspect.a033530. Cold Spring Harb Perspect Med. 2019. PMID: 30082454 Free PMC article. Review.
-
Genetic tools for studying cochlear inhibition.Front Cell Neurosci. 2024 Mar 15;18:1372948. doi: 10.3389/fncel.2024.1372948. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38560293 Free PMC article. Review.
-
A 'calcium capacitor' shapes cholinergic inhibition of cochlear hair cells.J Physiol. 2014 Aug 15;592(16):3393-401. doi: 10.1113/jphysiol.2013.267914. Epub 2014 Feb 24. J Physiol. 2014. PMID: 24566542 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

