Recombinant Bacillus subtilis that grows on untreated plant biomass
- PMID: 23183968
- PMCID: PMC3568581
- DOI: 10.1128/AEM.02433-12
Recombinant Bacillus subtilis that grows on untreated plant biomass
Retraction in
-
Retraction for Anderson et al., Recombinant Bacillus subtilis That Grows on Untreated Plant Biomass.Appl Environ Microbiol. 2015 Nov;81(22):7957. doi: 10.1128/AEM.02768-15. Appl Environ Microbiol. 2015. PMID: 26487766 Free PMC article. No abstract available.
Abstract
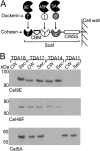
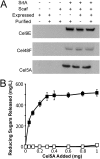
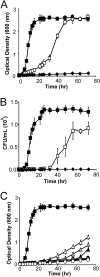
Lignocellulosic biomass is a promising feedstock to produce biofuels and other valuable biocommodities. A major obstacle to its commercialization is the high cost of degrading biomass into fermentable sugars, which is typically achieved using cellulolytic enzymes from Trichoderma reesei. Here, we explore the use of microbes to break down biomass. Bacillus subtilis was engineered to display a multicellulase-containing minicellulosome. The complex contains a miniscaffoldin protein that is covalently attached to the cell wall and three noncovalently associated cellulase enzymes derived from Clostridium cellulolyticum (Cel48F, Cel9E, and Cel5A). The minicellulosome spontaneously assembles, thus increasing the practicality of the cells. The recombinant bacteria are highly cellulolytic and grew in minimal medium containing industrially relevant forms of biomass as the primary nutrient source (corn stover, hatched straw, and switch grass). Notably, growth did not require dilute acid pretreatment of the biomass and the cells achieved densities approaching those of cells cultured with glucose. An analysis of the sugars released from acid-pretreated corn stover indicates that the cells have stable cellulolytic activity that enables them to break down 62.3% ± 2.6% of the biomass. When supplemented with beta-glucosidase, the cells liberated 21% and 33% of the total available glucose and xylose in the biomass, respectively. As the cells display only three types of enzymes, increasing the number of displayed enzymes should lead to even more potent cellulolytic microbes. This work has important implications for the efficient conversion of lignocellulose to value-added biocommodities.
Figures



References
-
- Kerr RA. 2008. Energy. World oil crunch looming? Science 322:1178–1179 - PubMed
-
- Boerjan W, Ralph J, Baucher M. 2003. Lignin biosynthesis. Annu. Rev. Plant Biol. 54:519–546 - PubMed
-
- Reddy N, Yang Y. 2005. Biofibers from agricultural byproducts for industrial applications. Trends Biotechnol. 23:22–27 - PubMed
-
- Werpy T, Petersen G. (ed). 2004. Top value added chemicals from biomass, vol 1. Results of screening for potential candidates from sugars and synthesis gas. Office of Scientific and Technical Information, US Department of Energy, Oak Ridge, TN
-
- Lynd LR, van Zyl WH, McBride JE, Laser M. 2005. Consolidated bioprocessing of cellulosic biomass: an update. Curr. Opin. Biotechnol. 16:577–583 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

