Inhibition of Staphylococcus aureus invasion into bovine mammary epithelial cells by contact with live Lactobacillus casei
- PMID: 23183972
- PMCID: PMC3568545
- DOI: 10.1128/AEM.03323-12
Inhibition of Staphylococcus aureus invasion into bovine mammary epithelial cells by contact with live Lactobacillus casei
Abstract
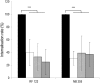
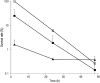
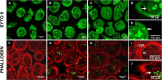
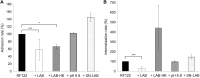
Staphylococcus aureus is a major pathogen that is responsible for mastitis in dairy herds. S. aureus mastitis is difficult to treat and prone to recurrence despite antibiotic treatment. The ability of S. aureus to invade bovine mammary epithelial cells (bMEC) is evoked to explain this chronicity. One sustainable alternative to treat or prevent mastitis is the use of lactic acid bacteria (LAB) as mammary probiotics. In this study, we tested the ability of Lactobacillus casei strains to prevent invasion of bMEC by two S. aureus bovine strains, RF122 and Newbould305, which reproducibly induce acute and moderate mastitis, respectively. L. casei strains affected adhesion and/or internalization of S. aureus in a strain-dependent manner. Interestingly, L. casei CIRM-BIA 667 reduced S. aureus Newbould305 and RF122 internalization by 60 to 80%, and this inhibition was confirmed for two other L. casei strains, including one isolated from bovine teat canal. The protective effect occurred without affecting bMEC morphology and viability. Once internalized, the fate of S. aureus was not affected by L. casei. It should be noted that L. casei was internalized at a low rate but survived in bMEC cells with a better efficiency than that of S. aureus RF122. Inhibition of S. aureus adhesion was maintained with heat-killed L. casei, whereas contact between live L. casei and S. aureus or bMEC was required to prevent S. aureus internalization. This first study of the antagonism of LAB toward S. aureus in a mammary context opens avenues for the development of novel control strategies against this major pathogen.
Figures



References
-
- Akers RM, Nickerson SC. 2011. Mastitis and its impact on structure and function in the ruminant mammary gland. J. Mammary Gland Biol. Neoplasia 16:275–289 - PubMed
-
- Archer GL. 1998. Staphylococcus aureus: a well-armed pathogen. Clin. Infect. Dis. 26:1179–1181 - PubMed
-
- Delgado S, Garcia P, Fernandez L, Jimenez E, Rodriguez-Banos M, Del CR, Rodriguez JM. 2011. Characterization of Staphylococcus aureus strains involved in human and bovine mastitis. FEMS Immunol. Med. Microbiol. 62:225–235 - PubMed
-
- Contreras GA, Rodriguez JM. 2011. Mastitis: comparative etiology and epidemiology. J. Mammary Gland Biol. Neoplasia 16:339–356 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

