Metabolic engineering of Synechocystis sp. strain PCC 6803 for isobutanol production
- PMID: 23183979
- PMCID: PMC3568544
- DOI: 10.1128/AEM.02827-12
Metabolic engineering of Synechocystis sp. strain PCC 6803 for isobutanol production
Abstract
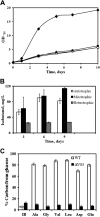
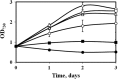
Global warming and decreasing fossil fuel reserves have prompted great interest in the synthesis of advanced biofuels from renewable resources. In an effort to address these concerns, we performed metabolic engineering of the cyanobacterium Synechocystis sp. strain PCC 6803 to develop a strain that can synthesize isobutanol under both autotrophic and mixotrophic conditions. With the expression of two heterologous genes from the Ehrlich pathway, the engineered strain can accumulate 90 mg/liter of isobutanol from 50 mM bicarbonate in a gas-tight shaking flask. The strain does not require any inducer (i.e., isopropyl β-d-1-thiogalactopyranoside [IPTG]) or antibiotics to maintain its isobutanol production. In the presence of glucose, isobutanol synthesis is only moderately promoted (titer = 114 mg/liter). Based on isotopomer analysis, we found that, compared to the wild-type strain, the mutant significantly reduced its glucose utilization and mainly employed autotrophic metabolism for biomass growth and isobutanol production. Since isobutanol is toxic to the cells and may also be degraded photochemically by hydroxyl radicals during the cultivation process, we employed in situ removal of the isobutanol using oleyl alcohol as a solvent trap. This resulted in a final net concentration of 298 mg/liter of isobutanol under mixotrophic culture conditions.
Figures

References
-
- Rittmann BE. 2008. Opportunities for renewable bioenergy using microorganisms. Biotechnol. Bioeng. 100:203–212 - PubMed
-
- van Groenigen KJ, Osenberg CW, Hungate BA. 2011. Increased soil emissions of potent greenhouse gases under increased atmospheric CO2. Nature 475:214–216 - PubMed
-
- Keasling JD, Chou H. 2008. Metabolic engineering delivers next-generation biofuels. Nat. Biotechnol. 26:298–299 - PubMed
-
- Atsumi S, Hanai T, Liao JC. 2008. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 451:86–89 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

