Zic3 is required in the extra-cardiac perinodal region of the lateral plate mesoderm for left-right patterning and heart development
- PMID: 23184148
- PMCID: PMC3606008
- DOI: 10.1093/hmg/dds494
Zic3 is required in the extra-cardiac perinodal region of the lateral plate mesoderm for left-right patterning and heart development
Abstract
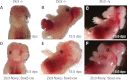
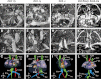
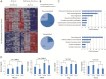
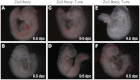
Mutations in ZIC3 cause human X-linked heterotaxy and isolated cardiovascular malformations. A mouse model with targeted deletion of Zic3 demonstrates an early role for Zic3 in gastrulation, CNS, cardiac and left-right axial development. The observation of multiple malformations in Zic3(null) mice and the relatively broad expression pattern of Zic3 suggest its important roles in multiple developmental processes. Here, we report that Zic3 is primarily required in epiblast derivatives to affect left-right patterning and its expression in epiblast is necessary for proper transcriptional control of embryonic cardiac development. However, cardiac malformations in Zic3 deficiency occur not because Zic3 is intrinsically required in the heart but rather because it functions early in the establishment of left-right body axis. In addition, we provide evidence supporting a role for Zic3 specifically in the perinodal region of the posterior lateral plate mesoderm for the establishment of laterality. These data delineate the spatial requirement of Zic3 during left-right patterning in the mammalian embryo, and provide basis for further understanding the molecular mechanisms underlying the complex interaction of Zic3 with signaling pathways involved in the early establishment of laterality.
Figures


Similar articles
-
Zic3 is required in the migrating primitive streak for node morphogenesis and left-right patterning.Hum Mol Genet. 2013 May 15;22(10):1913-23. doi: 10.1093/hmg/ddt001. Epub 2013 Jan 8. Hum Mol Genet. 2013. PMID: 23303524 Free PMC article.
-
Zic3 is involved in the left-right specification of the Xenopus embryo.Development. 2000 Nov;127(22):4787-95. doi: 10.1242/dev.127.22.4787. Development. 2000. PMID: 11044394
-
Zic3 is critical for early embryonic patterning during gastrulation.Dev Dyn. 2006 Mar;235(3):776-85. doi: 10.1002/dvdy.20668. Dev Dyn. 2006. PMID: 16397896
-
ZIC3 in Heterotaxy.Adv Exp Med Biol. 2018;1046:301-327. doi: 10.1007/978-981-10-7311-3_15. Adv Exp Med Biol. 2018. PMID: 29442328 Free PMC article. Review.
-
The role of ZIC3 in vertebrate development.Cytogenet Genome Res. 2002;99(1-4):229-35. doi: 10.1159/000071598. Cytogenet Genome Res. 2002. PMID: 12900569 Review.
Cited by
-
Genetic and functional analyses of ZIC3 variants in congenital heart disease.Hum Mutat. 2014 Jan;35(1):66-75. doi: 10.1002/humu.22457. Hum Mutat. 2014. PMID: 24123890 Free PMC article.
-
Embryology of the Abdominal Wall and Associated Malformations-A Review.Front Surg. 2022 Jul 7;9:891896. doi: 10.3389/fsurg.2022.891896. eCollection 2022. Front Surg. 2022. PMID: 35874129 Free PMC article. Review.
-
Multigenerational analysis of sex-specific phenotypic differences at midgestation caused by abnormal folate metabolism.Environ Epigenet. 2017 Nov 3;3(4):dvx014. doi: 10.1093/eep/dvx014. eCollection 2017 Oct. Environ Epigenet. 2017. PMID: 29492317 Free PMC article.
-
A CRISPR mis-insertion in the Zic3 5'UTR inhibits in vivo translation and is predicted to result in formation of an mRNA stem-loop hairpin.Biol Open. 2025 Mar 15;14(3):bio061677. doi: 10.1242/bio.061677. Epub 2025 Mar 17. Biol Open. 2025. PMID: 39912332 Free PMC article.
-
Human-gained heart enhancers are associated with species-specific cardiac attributes.Nat Cardiovasc Res. 2022 Sep;1(9):830-843. doi: 10.1038/s44161-022-00124-7. Epub 2022 Sep 15. Nat Cardiovasc Res. 2022. PMID: 36817700 Free PMC article.
References
-
- Aruga J., Nagai T., Tokuyama T., Hayashizaki Y., Okazaki Y., Chapman V.M., Mikoshiba K. The mouse zic gene family. Homologues of the Drosophila pair-rule gene odd-paired. J. Biol. Chem. 1996;271:1043–1047. doi:10.1074/jbc.271.2.1043. - DOI - PubMed
-
- Aruga J., Yokota N., Hashimoto M., Furuichi T., Fukuda M., Mikoshiba K. A novel zinc finger protein, zic, is involved in neurogenesis, especially in the cell lineage of cerebellar granule cells. J. Neurochem. 1994;63:1880–1890. doi:10.1046/j.1471-4159.1994.63051880.x. - DOI - PubMed
-
- Gebbia M., Ferrero G.B., Pilia G., Bassi M.T., Aylsworth A., Penman-Splitt M., Bird L.M., Bamforth J.S., Burn J., Schlessinger D., et al. X-linked situs abnormalities result from mutations in ZIC3. Nat. Genet. 1997;17:305–308. doi:10.1038/ng1197-305. - DOI - PubMed
-
- Zhu L., Belmont J.W., Ware S.M. Genetics of human heterotaxias. Eur. J. Hum. Genet. 2006;14:17–25. - PubMed
-
- Nakata K., Nagai T., Aruga J., Mikoshiba K. Xenopus Zic3, a primary regulator both in neural and neural crest development. Proc. Natl Acad. Sci. U.S.A. 1997;94:11980–11985. doi:10.1073/pnas.94.22.11980. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

