Nucleus-localized 21.5-kDa myelin basic protein promotes oligodendrocyte proliferation and enhances neurite outgrowth in coculture, unlike the plasma membrane-associated 18.5-kDa isoform
- PMID: 23184356
- PMCID: PMC3569502
- DOI: 10.1002/jnr.23166
Nucleus-localized 21.5-kDa myelin basic protein promotes oligodendrocyte proliferation and enhances neurite outgrowth in coculture, unlike the plasma membrane-associated 18.5-kDa isoform
Abstract
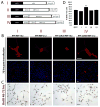
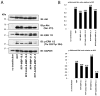
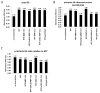
The classic myelin basic protein (MBP) family of central nervous system (CNS) myelin arises from transcription start site 3 of the Golli (gene of oligodendrocyte lineage) complex and comprises splice isoforms ranging in nominal molecular mass from 14 kDa to (full-length) 21.5 kDa. We have determined here a number of distinct functional differences between the major 18.5-kDa and minor 21.5-kDa isoforms of classic MBP with respect to oligodendrocyte (OLG) proliferation. We have found that, in contrast to 18.5-kDa MBP, 21.5-kDa MBP increases proliferation of early developmental immortalized N19-OLGs by elevating the levels of phosphorylated ERK1/2 and Akt1 kinases and of ribosomal protein S6. Coculture of N2a neuronal cells with N19-OLGs transfected with the 21.5-kDa isoform (or conditioned medium from), but not the 18.5-kDa isoform, caused the N2a cells to have increased neurite outgrowth and process branching complexity. These roles were dependent on subcellular localization of 21.5-kDa MBP to the nucleus and on the exon II-encoded segment, suggesting that the nuclear localization of early minor isoforms of MBP may play a crucial role in regulating and/or initiating myelin and neuronal development in the mammalian CNS.
Copyright © 2012 Wiley Periodicals, Inc.
Figures


Similar articles
-
Classic 18.5- and 21.5-kDa myelin basic protein isoforms associate with cytoskeletal and SH3-domain proteins in the immortalized N19-oligodendroglial cell line stimulated by phorbol ester and IGF-1.Neurochem Res. 2012 Jun;37(6):1277-95. doi: 10.1007/s11064-011-0700-2. Epub 2012 Jan 17. Neurochem Res. 2012. PMID: 22249765 Free PMC article.
-
Classical 18.5-and 21.5-kDa isoforms of myelin basic protein inhibit calcium influx into oligodendroglial cells, in contrast to golli isoforms.J Neurosci Res. 2011 Apr;89(4):467-80. doi: 10.1002/jnr.22570. Epub 2011 Jan 13. J Neurosci Res. 2011. PMID: 21312222 Free PMC article.
-
Interaction of myelin basic protein with cytoskeletal and signaling proteins in cultured primary oligodendrocytes and N19 oligodendroglial cells.BMC Res Notes. 2014 Jun 24;7:387. doi: 10.1186/1756-0500-7-387. BMC Res Notes. 2014. PMID: 24956930 Free PMC article.
-
Myelin management by the 18.5-kDa and 21.5-kDa classic myelin basic protein isoforms.J Neurochem. 2013 May;125(3):334-61. doi: 10.1111/jnc.12195. Epub 2013 Mar 6. J Neurochem. 2013. PMID: 23398367 Free PMC article. Review.
-
MyelStones: the executive roles of myelin basic protein in myelin assembly and destabilization in multiple sclerosis.Biochem J. 2015 Nov 15;472(1):17-32. doi: 10.1042/BJ20150710. Biochem J. 2015. PMID: 26518750 Review.
Cited by
-
Lentiviral Vector-Mediated p27kip1 Expression Facilitates Recovery After Spinal Cord Injury.Mol Neurobiol. 2016 Nov;53(9):6043-6056. doi: 10.1007/s12035-015-9498-2. Epub 2015 Nov 2. Mol Neurobiol. 2016. PMID: 26526846
-
Comparative profiling of white matter development in the human and mouse brain reveals volumetric deficits and delayed myelination in Angelman syndrome.Mol Autism. 2024 Dec 26;15(1):54. doi: 10.1186/s13229-024-00636-y. Mol Autism. 2024. PMID: 39726042 Free PMC article.
-
Comparative profiling of white matter development in the human and mouse brain reveals volumetric deficits and delayed myelination in Angelman syndrome.Res Sq [Preprint]. 2024 Aug 9:rs.3.rs-4681861. doi: 10.21203/rs.3.rs-4681861/v1. Res Sq. 2024. Update in: Mol Autism. 2024 Dec 26;15(1):54. doi: 10.1186/s13229-024-00636-y. PMID: 39149488 Free PMC article. Updated. Preprint.
-
Prenatal stress-induced programming of genome-wide promoter DNA methylation in 5-HTT-deficient mice.Transl Psychiatry. 2014 Oct 21;4(10):e473. doi: 10.1038/tp.2014.107. Transl Psychiatry. 2014. PMID: 25335169 Free PMC article.
-
Making myelin basic protein -from mRNA transport to localized translation.Front Cell Neurosci. 2013 Sep 27;7:169. doi: 10.3389/fncel.2013.00169. Front Cell Neurosci. 2013. PMID: 24098271 Free PMC article. Review.
References
-
- Aggarwal S, Yurlova L, Simons M. Central nervous system myelin: structure, synthesis and assembly. Trends Cell Biol. 2011a;21:585–593. - PubMed
-
- Aggarwal S, Yurlova L, Snaidero N, Reetz C, Frey S, Zimmermann J, Pahler G, Janshoff A, Friedrichs J, Muller DJ, Goebel C, Simons M. A size barrier limits protein diffusion at the cell surface to generate lipid-rich myelin-membrane sheets. Dev Cell. 2011b;21:445–456. - PubMed
-
- Baccarini M. Second nature: biological functions of the Raf-1 “kinase. FEBS Lett. 2005;579:3271–3277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

