Selective inhibition of Cx43 hemichannels by Gap19 and its impact on myocardial ischemia/reperfusion injury
- PMID: 23184389
- PMCID: PMC3666173
- DOI: 10.1007/s00395-012-0309-x
Selective inhibition of Cx43 hemichannels by Gap19 and its impact on myocardial ischemia/reperfusion injury
Abstract
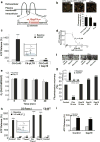
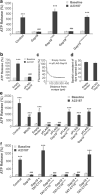
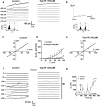
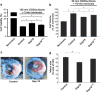
Connexin-43 (Cx43), a predominant cardiac connexin, forms gap junctions (GJs) that facilitate electrical cell-cell coupling and unapposed/nonjunctional hemichannels that provide a pathway for the exchange of ions and metabolites between cytoplasm and extracellular milieu. Uncontrolled opening of hemichannels in the plasma membrane may be deleterious for the myocardium and blocking hemichannels may confer cardioprotection by preventing ionic imbalance, cell swelling and loss of critical metabolites. Currently, all known hemichannel inhibitors also block GJ channels, thereby disturbing electrical cell-cell communication. Here we aimed to characterize a nonapeptide, called Gap19, derived from the cytoplasmic loop (CL) of Cx43 as a hemichannel blocker and examined its effect on hemichannel currents in cardiomyocytes and its influence in cardiac outcome after ischemia/reperfusion. We report that Gap 19 inhibits Cx43 hemichannels without blocking GJ channels or Cx40/pannexin-1 hemichannels. Hemichannel inhibition is due to the binding of Gap19 to the C-terminus (CT) thereby preventing intramolecular CT-CL interactions. The peptide inhibited Cx43 hemichannel unitary currents in both HeLa cells exogenously expressing Cx43 and acutely isolated pig ventricular cardiomyocytes. Treatment with Gap19 prevented metabolic inhibition-enhanced hemichannel openings, protected cardiomyocytes against volume overload and cell death following ischemia/reperfusion in vitro and modestly decreased the infarct size after myocardial ischemia/reperfusion in mice in vivo. We conclude that preventing Cx43 hemichannel opening with Gap19 confers limited protective effects against myocardial ischemia/reperfusion injury.
Conflict of interest statement
Figures


References
-
- Boengler K, Dodoni G, Rodriguez-Sinovas A, Cabestrero A, Ruiz-Meana M, Gres P, Konietzka I, Lopez-Iglesias C, Garcia-Dorado D, Di LF, Heusch G, Schulz R. Connexin 43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovasc Res. 2005;67:234–244. doi: 10.1016/j.cardiores.2005.04.014. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

