Application of PBPK modeling to predict monoclonal antibody disposition in plasma and tissues in mouse models of human colorectal cancer
- PMID: 23184417
- PMCID: PMC3727051
- DOI: 10.1007/s10928-012-9279-8
Application of PBPK modeling to predict monoclonal antibody disposition in plasma and tissues in mouse models of human colorectal cancer
Abstract
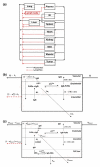
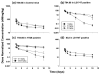
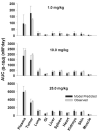
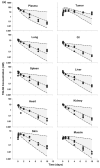
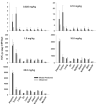
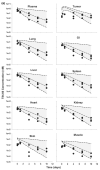
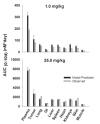
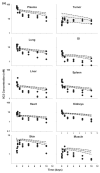
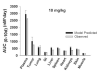
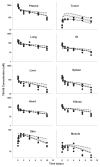
This investigation evaluated the utility of a physiologically based pharmacokinetic (PBPK) model, which incorporates model parameters representing key determinants of monoclonal antibody (mAb) target-mediated disposition, to predict, a priori, mAb disposition in plasma and in tissues, including tumors that express target antigens. Monte Carlo simulation techniques were employed to predict the disposition of two mAbs, 8C2 (as a non-binding control mouse IgG1 mAb) and T84.66 (a high-affinity murine IgG1 anti-carcinoembryonic antigen mAb), in mice bearing no tumors, or bearing colorectal HT29 or LS174T xenografts. Model parameters were obtained or derived from the literature. (125)I-T84.66 and (125)I-8C2 were administered to groups of SCID mice, and plasma and tissue concentrations were determined via gamma counting. The PBPK model well-predicted the experimental data. Comparisons of the population predicted versus observed areas under the plasma concentration versus time curve (AUC) for T84.66 were 95.4 ± 67.8 versus 84.0 ± 3.0, 1,859 ± 682 versus 2,370 ± 154, and 5,930 ± 1,375 versus 5,960 ± 317 (nM × day) at 1, 10, and 25 mg/kg in LS174T xenograft-bearing SCID mice; and 215 ± 72 versus 233 ± 30, 3,070 ± 346 versus 3,120 ± 180, and 7,884 ± 714 versus 7,440 ± 626 in HT29 xenograft-bearing mice. Model predicted versus observed 8C2 plasma AUCs were 312.4 ± 30 versus 182 ± 7.6 and 7,619 ± 738 versus 7,840 ± 24.3 (nM × day) at 1 and 25 mg/kg. High correlations were observed between the predicted median plasma concentrations and observed median plasma concentrations (r (2) = 0.927, for all combinations of treatment, dose, and tumor model), highlighting the utility of the PBPK model for the a priori prediction of in vivo data.
Figures







Similar articles
-
Physiologically Based Modeling of the Pharmacokinetics of "Catch-and-Release" Anti-Carcinoembryonic Antigen Monoclonal Antibodies in Colorectal Cancer Xenograft Mouse Models.J Pharm Sci. 2019 Jan;108(1):674-691. doi: 10.1016/j.xphs.2018.09.037. Epub 2018 Oct 12. J Pharm Sci. 2019. PMID: 30321546 Free PMC article.
-
Dynamic Contrast-Enhanced Magnetic Resonance Imaging for the Prediction of Monoclonal Antibody Tumor Disposition.Int J Mol Sci. 2022 Jan 8;23(2):679. doi: 10.3390/ijms23020679. Int J Mol Sci. 2022. PMID: 35054865 Free PMC article.
-
Pharmacokinetic mAb-mAb interaction: anti-VEGF mAb decreases the distribution of anti-CEA mAb into colorectal tumor xenografts.AAPS J. 2012 Sep;14(3):445-55. doi: 10.1208/s12248-012-9357-2. Epub 2012 Apr 18. AAPS J. 2012. PMID: 22528507 Free PMC article.
-
Projecting human pharmacokinetics of monoclonal antibodies from nonclinical data: comparative evaluation of prediction approaches in early drug development.Biopharm Drug Dispos. 2016 Mar;37(2):51-65. doi: 10.1002/bdd.1952. Epub 2015 May 15. Biopharm Drug Dispos. 2016. PMID: 25869767 Review.
-
Physiologically-based modeling of monoclonal antibody pharmacokinetics in drug discovery and development.Drug Metab Pharmacokinet. 2019 Feb;34(1):3-13. doi: 10.1016/j.dmpk.2018.11.002. Epub 2018 Nov 22. Drug Metab Pharmacokinet. 2019. PMID: 30522890 Free PMC article. Review.
Cited by
-
Image-guided mathematical modeling for pharmacological evaluation of nanomaterials and monoclonal antibodies.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020 Sep;12(5):e1628. doi: 10.1002/wnan.1628. Epub 2020 Apr 21. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020. PMID: 32314552 Free PMC article. Review.
-
Second-generation minimal physiologically-based pharmacokinetic model for monoclonal antibodies.J Pharmacokinet Pharmacodyn. 2013 Oct;40(5):597-607. doi: 10.1007/s10928-013-9332-2. Epub 2013 Aug 31. J Pharmacokinet Pharmacodyn. 2013. PMID: 23996115 Free PMC article.
-
A minimal physiologically based pharmacokinetic model to study the combined effect of antibody size, charge, and binding affinity to FcRn/antigen on antibody pharmacokinetics.J Pharmacokinet Pharmacodyn. 2024 Oct;51(5):477-492. doi: 10.1007/s10928-023-09899-z. Epub 2024 Feb 24. J Pharmacokinet Pharmacodyn. 2024. PMID: 38400996 Free PMC article.
-
Review of the Existing Translational Pharmacokinetics Modeling Approaches Specific to Monoclonal Antibodies (mAbs) to Support the First-In-Human (FIH) Dose Selection.Int J Mol Sci. 2022 Oct 22;23(21):12754. doi: 10.3390/ijms232112754. Int J Mol Sci. 2022. PMID: 36361546 Free PMC article. Review.
-
MPBPK-TMDD models for mAbs: alternative models, comparison, and identifiability issues.J Pharmacokinet Pharmacodyn. 2018 Dec;45(6):787-802. doi: 10.1007/s10928-018-9608-7. Epub 2018 Nov 10. J Pharmacokinet Pharmacodyn. 2018. PMID: 30415351
References
-
- Yan L, Hsu K, Beckman RA. Antibody-based therapy for solid tumors. Cancer J. 2008;14:178–183. - PubMed
-
- Jain RK. Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res. 1990;50:814s–819s. - PubMed
-
- Jain RK. Tumor physiology and antibody delivery. Front Radiat Ther Oncol. 1990;24:32–46. - PubMed
-
- Fujimori K, Covell DG, Fletcher JE, Weinstein JN. A modeling analysis of monoclonal antibody percolation through tumors: a binding-site barrier. J Nucl Med. 1990;31:1191–1198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

