Synchronization and desynchronization in epilepsy: controversies and hypotheses
- PMID: 23184516
- PMCID: PMC3591697
- DOI: 10.1113/jphysiol.2012.239590
Synchronization and desynchronization in epilepsy: controversies and hypotheses
Abstract
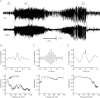
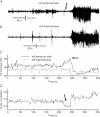
Epilepsy has been historically seen as a functional brain disorder associated with excessive synchronization of large neuronal populations leading to a hypersynchronous state. Recent evidence showed that epileptiform phenomena, particularly seizures, result from complex interactions between neuronal networks characterized by heterogeneity of neuronal firing and dynamical evolution of synchronization. Desynchronization is often observed preceding seizures or during their early stages; in contrast, high levels of synchronization observed towards the end of seizures may facilitate termination. In this review we discuss cellular and network mechanisms responsible for such complex changes in synchronization. Recent work has identified cell-type-specific inhibitory and excitatory interactions, the dichotomy between neuronal firing and the non-local measurement of local field potentials distant to that firing, and the reflection of the neuronal dark matter problem in non-firing neurons active in seizures. These recent advances have challenged long-established views and are leading to a more rigorous and realistic understanding of the pathophysiology of epilepsy.
Figures


References
-
- Babb TL, Wilson CL, Isokawa-Akesson M. Firing patterns of human limbic neurons during stereoencephalography (SEEG) and clinical temporal lobe seizures. Electroencephalogr Clin Neurophysiol. 1987;66:467–482. - PubMed
-
- Bikson M, Fox JE, Jefferys JGR. Neuronal aggregate formation underlies spatiotemporal dynamics of nonsynaptic seizure initiation. J Neurophysiol. 2003;89:2330–2333. - PubMed
-
- Bishop G. Cyclic changes in excitability of the optic pathway of the rabbit. American Journal of Physiology-Legacy. 1932;103:213–224.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

