The 3-hydroxy-methylglutaryl coenzyme A lyase HCL1 is required for macrophage colonization by human fungal pathogen Histoplasma capsulatum
- PMID: 23184522
- PMCID: PMC3553812
- DOI: 10.1128/IAI.00833-12
The 3-hydroxy-methylglutaryl coenzyme A lyase HCL1 is required for macrophage colonization by human fungal pathogen Histoplasma capsulatum
Abstract
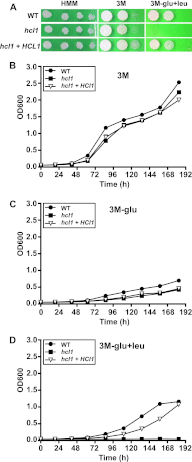
Histoplasma capsulatum is a fungal respiratory pathogen that survives and replicates within the phagolysosome of macrophages. The molecular factors it utilizes to subvert macrophage antimicrobial defenses are largely unknown. Although the ability of H. capsulatum to prevent acidification of the macrophage phagolysosome is thought to be critical for intracellular survival, this hypothesis has not been tested since H. capsulatum mutants that experience decreased phagosomal pH have not been identified. In a screen to identify H. capsulatum genes required for lysis of bone marrow-derived macrophages (BMDMs), we identified an insertion mutation disrupting the H. capsulatum homolog of 3-hydroxy-methylglutaryl coenzyme A (HMG CoA) lyase (HCL1). In addition to its inability to lyse macrophages, the hcl1 mutant had a severe growth defect in BMDMs, indicating that HMG CoA lyase gene function is critical for macrophage colonization. In other organisms, HMG CoA lyase catalyzes the last step in the leucine catabolism pathway. In addition, both fungi and humans deficient in HMG CoA lyase accumulate acidic intermediates as a consequence of their inability to catabolize leucine. Consistent with observations in other organisms, the H. capsulatum hcl1 mutant was unable to grow on leucine as the major carbon source, caused acidification of its growth medium in vitro, and resided in an acidified vacuole within macrophages. Mice infected with the hcl1 mutant took significantly longer to succumb to infection than mice infected with the wild-type strain. Taken together, these data indicate the importance of Hcl1 function in H. capsulatum replication in the harsh growth environment of the macrophage phagosome.
Figures





Similar articles
-
3-Hydroxy-3-methylglutaryl-coenzyme A lyase deficiency: initial presentation in a young adult.J Inherit Metab Dis. 2009 Dec;32 Suppl 1:S49-52. doi: 10.1007/s10545-009-1048-5. Epub 2009 Feb 24. J Inherit Metab Dis. 2009. PMID: 19242819
-
The Histoplasma capsulatum vacuolar ATPase is required for iron homeostasis, intracellular replication in macrophages and virulence in a murine model of histoplasmosis.Mol Microbiol. 2008 Oct;70(1):127-39. doi: 10.1111/j.1365-2958.2008.06395.x. Epub 2008 Aug 11. Mol Microbiol. 2008. PMID: 18699866 Free PMC article.
-
Metabolism of Gluconeogenic Substrates by an Intracellular Fungal Pathogen Circumvents Nutritional Limitations within Macrophages.mBio. 2020 Apr 7;11(2):e02712-19. doi: 10.1128/mBio.02712-19. mBio. 2020. PMID: 32265333 Free PMC article.
-
Knocking on the right door and making a comfortable home: Histoplasma capsulatum intracellular pathogenesis.Curr Opin Microbiol. 2003 Aug;6(4):327-31. doi: 10.1016/s1369-5274(03)00080-8. Curr Opin Microbiol. 2003. PMID: 12941399 Review.
-
Iron uptake and virulence in Histoplasma capsulatum.Curr Opin Microbiol. 2013 Dec;16(6):700-7. doi: 10.1016/j.mib.2013.09.001. Epub 2013 Oct 1. Curr Opin Microbiol. 2013. PMID: 24094809 Review.
Cited by
-
Kinase Inhibitor Library Screening Identifies the Cancer Therapeutic Sorafenib and Structurally Similar Compounds as Strong Inhibitors of the Fungal Pathogen Histoplasma capsulatum.Antibiotics (Basel). 2021 Oct 8;10(10):1223. doi: 10.3390/antibiotics10101223. Antibiotics (Basel). 2021. PMID: 34680804 Free PMC article.
-
Thermally Dimorphic Human Fungal Pathogens--Polyphyletic Pathogens with a Convergent Pathogenicity Trait.Cold Spring Harb Perspect Med. 2014 Nov 10;5(8):a019794. doi: 10.1101/cshperspect.a019794. Cold Spring Harb Perspect Med. 2014. PMID: 25384771 Free PMC article. Review.
-
The transcription factor CHOP, an effector of the integrated stress response, is required for host sensitivity to the fungal intracellular pathogen Histoplasma capsulatum.PLoS Pathog. 2017 Sep 27;13(9):e1006589. doi: 10.1371/journal.ppat.1006589. eCollection 2017 Sep. PLoS Pathog. 2017. PMID: 28953979 Free PMC article.
-
Pathogenicity & virulence of Histoplasma capsulatum - A multifaceted organism adapted to intracellular environments.Virulence. 2022 Dec;13(1):1900-1919. doi: 10.1080/21505594.2022.2137987. Virulence. 2022. PMID: 36266777 Free PMC article. Review.
-
Histoplasmosis in Solid Organ Transplantation.J Fungi (Basel). 2024 Feb 2;10(2):124. doi: 10.3390/jof10020124. J Fungi (Basel). 2024. PMID: 38392796 Free PMC article. Review.
References
-
- Cano MV, Hajjeh RA. 2001. The epidemiology of histoplasmosis: a review. Semin. Respir. Infect. 16:109–118 - PubMed
-
- Wolf JE, Kerchberger V, Kobayashi GS, Little JR. 1987. Modulation of the macrophage oxidative burst by Histoplasma capsulatum. J. Immunol. 138:582–586 - PubMed
-
- Strasser JE, Newman SL, Ciraolo GM, Morris RE, Howell ML, Dean GE. 1999. Regulation of the macrophage vacuolar ATPase and phagosome-lysosome fusion by Histoplasma capsulatum. J. Immunol. 162:6148–6154 - PubMed

