M-ficolin binds selectively to the capsular polysaccharides of Streptococcus pneumoniae serotypes 19B and 19C and of a Streptococcus mitis strain
- PMID: 23184524
- PMCID: PMC3553806
- DOI: 10.1128/IAI.01148-12
M-ficolin binds selectively to the capsular polysaccharides of Streptococcus pneumoniae serotypes 19B and 19C and of a Streptococcus mitis strain
Erratum in
- Infect Immun. 2013 Nov;81(11):4321
Abstract
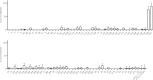
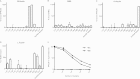
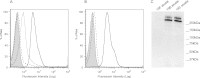
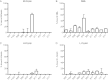
The three human ficolins (H-, L-, and M-ficolins) and mannan-binding lectin are pattern recognition molecules of the innate immune system mediating activation of the lectin pathway of the complement system. These four human proteins bind to some microorganisms and may be involved in the resolution of infections. We investigated binding selectivity by examining the binding of M-ficolin to a panel of more than 100 different streptococcal strains (Streptococcus pneumoniae and Streptococcus mitis), each expressing distinct polysaccharide structures. M-ficolin binding was observed for three strains only: strains of the pneumococcal serotypes 19B and 19C and a single S. mitis strain expressing a similar polysaccharide structure. The bound M-ficolin, in association with MASP-2, mediated the cleavage of complement factor C4. Binding to the bacteria was inhibitable by N-acetylglucosamine, indicating that the interaction with the bacterial surface takes place via the fibrinogen-like domain. The common N-acetylmannosamine residue present in the structures of the four capsular polysaccharides of group 19 is linked via a phosphodiester bond. This residue is apparently not a ligand for M-ficolin, since the lectin binds to two of the group 19 polysaccharides only. M-ficolin bound strongly to serotype 19B and 19C polysaccharides. In contrast to those of serotypes 19A and 19F, serotype 19B and 19C polysaccharides contain an extra N-acetylmannosamine residue linked via glycoside linkage only. Thus, this extra residue seems to be the M-ficolin ligand. In conclusion, we were able to demonstrate specific binding of M-ficolin to some capsular polysaccharides of the opportunistic pathogen S. pneumoniae and of the commensal bacterium S. mitis.
Figures



Similar articles
-
Low invasiveness of pneumococcal serotype 11A is linked to ficolin-2 recognition of O-acetylated capsule epitopes and lectin complement pathway activation.J Infect Dis. 2014 Oct 1;210(7):1155-65. doi: 10.1093/infdis/jiu195. Epub 2014 Mar 27. J Infect Dis. 2014. PMID: 24683196 Free PMC article.
-
L-Ficolin/mannose-binding lectin-associated serine protease complexes bind to group B streptococci primarily through N-acetylneuraminic acid of capsular polysaccharide and activate the complement pathway.Infect Immun. 2008 Jan;76(1):179-88. doi: 10.1128/IAI.00837-07. Epub 2007 Oct 15. Infect Immun. 2008. PMID: 17938215 Free PMC article.
-
The lectin pathway of complement activation is a critical component of the innate immune response to pneumococcal infection.PLoS Pathog. 2012;8(7):e1002793. doi: 10.1371/journal.ppat.1002793. Epub 2012 Jul 5. PLoS Pathog. 2012. PMID: 22792067 Free PMC article.
-
Structural and functional overview of the lectin complement pathway: its molecular basis and physiological implication.Arch Immunol Ther Exp (Warsz). 2013 Aug;61(4):273-83. doi: 10.1007/s00005-013-0229-y. Epub 2013 Apr 7. Arch Immunol Ther Exp (Warsz). 2013. PMID: 23563865 Review.
-
The role of ficolins in the lectin pathway of innate immunity.Int J Biochem Cell Biol. 2011 May;43(5):705-12. doi: 10.1016/j.biocel.2011.02.003. Epub 2011 Feb 18. Int J Biochem Cell Biol. 2011. PMID: 21315829 Review.
Cited by
-
Intranasal Immunization with the Commensal Streptococcus mitis Confers Protective Immunity against Pneumococcal Lung Infection.Appl Environ Microbiol. 2019 Mar 6;85(6):e02235-18. doi: 10.1128/AEM.02235-18. Print 2019 Mar 15. Appl Environ Microbiol. 2019. PMID: 30683742 Free PMC article.
-
Sugar-Coated Killer: Serotype 3 Pneumococcal Disease.Front Cell Infect Microbiol. 2020 Dec 23;10:613287. doi: 10.3389/fcimb.2020.613287. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33425786 Free PMC article. Review.
-
Low invasiveness of pneumococcal serotype 11A is linked to ficolin-2 recognition of O-acetylated capsule epitopes and lectin complement pathway activation.J Infect Dis. 2014 Oct 1;210(7):1155-65. doi: 10.1093/infdis/jiu195. Epub 2014 Mar 27. J Infect Dis. 2014. PMID: 24683196 Free PMC article.
-
A recombinant horseshoe crab plasma lectin recognizes specific pathogen-associated molecular patterns of bacteria through rhamnose.PLoS One. 2014 Dec 26;9(12):e115296. doi: 10.1371/journal.pone.0115296. eCollection 2014. PLoS One. 2014. PMID: 25541995 Free PMC article.
-
Interaction of lectin pathway of complement-activating pattern recognition molecules with mycobacteria.Clin Exp Immunol. 2014 Nov;178(2):310-9. doi: 10.1111/cei.12416. Clin Exp Immunol. 2014. PMID: 25041480 Free PMC article.
References
-
- Thiel S. 2007. Complement activating soluble pattern recognition molecules with collagen-like regions, mannan-binding lectin, ficolins and associated proteins. Mol. Immunol. 44:3875–3888 - PubMed
-
- Keshi H, Sakamoto T, Kawai T, Ohtani K, Katoh T, Jang SJ, Motomura W, Yoshizaki T, Fukuda M, Koyama S, Fukuzawa J, Fukuoh A, Yoshida I, Suzuki Y, Wakamiya N. 2006. Identification and characterization of a novel human collectin CL-K1. Microbiol. Immunol. 50:1001–1013 - PubMed
-
- Hansen S, Selman L, Palaniyar N, Ziegler K, Brandt J, Kliem A, Jonasson M, Skjoedt MO, Nielsen O, Hartshorn K, Jorgensen TJ, Skjodt K, Holmskov U. 2010. Collectin 11 (CL-11, CL-K1) is a MASP-1/3-associated plasma collectin with microbial-binding activity. J. Immunol. 185:6096–6104 - PubMed
-
- Ali YM, Lynch NJ, Haleem KS, Fujita T, Endo Y, Hansen S, Holmskov U, Takahashi K, Stahl GL, Dudler T, Girija UV, Wallis R, Kadioglu A, Stover CM, Andrew PW, Schwaeble WJ. 2012. The lectin pathway of complement activation is a critical component of the innate immune response to pneumococcal infection. PLoS Pathog. 8:e1002793 doi:10.1371/journal.ppat.1002793 - DOI - PMC - PubMed
-
- Endo Y, Sato Y, Matsushita M, Fujita T. 1996. Cloning and characterization of the human lectin P35 gene and its related gene. Genomics 36:515–521 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

