A technique to increase accessibility to late-stage chick embryos for in ovo manipulations
- PMID: 23184557
- PMCID: PMC3553263
- DOI: 10.1002/dvdy.23907
A technique to increase accessibility to late-stage chick embryos for in ovo manipulations
Abstract
Background: During early development, avian embryos are easily accessible in ovo for transplantations and experimental perturbations. However, these qualities of the avian embryonic model rapidly wane shortly after embryonic day (E)4 when the embryo is obscured by extraembryonic membranes, making it difficult to study developmental events that occur at later stages in vivo.
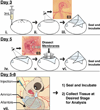
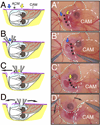
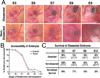
Results: In this study, we describe a multistep method that involves initially windowing eggs at E3, followed by dissecting away extraembryonic membranes at E5 to facilitate embryo accessibility in ovo until later stages of development. The majority of the embryos subjected to this technique remain exposed between E5 and E8, then become gradually displaced by the growing allantois from posterior to anterior regions.
Conclusions: Exposed embryos are viable and compatible with embryological and modern developmental biology techniques including tissue grafting and ablation, gene manipulation by electroporation, and protein expression. This technique opens up new avenues for studying complex cellular interactions during organogenesis and can be further extrapolated to regeneration and stem cell studies.
Copyright © 2012 Wiley Periodicals, Inc.
Figures


Similar articles
-
Surgical manipulation of the developing chick embryo in ovo after the 4th day of incubation.Lab Anim. 1983 Apr;17(2):123-4. doi: 10.1258/002367783780959466. Lab Anim. 1983. PMID: 6865318
-
Optimized ex-ovo culturing of chick embryos to advanced stages of development.J Vis Exp. 2015 Jan 24;(95):52129. doi: 10.3791/52129. J Vis Exp. 2015. PMID: 25650550 Free PMC article.
-
The method of chicken whole embryo culture using the eggshell windowing, surrogate eggshell and ex ovo culture system.Br Poult Sci. 2018 Apr;59(2):240-244. doi: 10.1080/00071668.2017.1413234. Epub 2018 Jan 11. Br Poult Sci. 2018. PMID: 29206486 Review.
-
Analysis of neural crest migration and differentiation by cross-species transplantation.J Vis Exp. 2012 Feb 7;(60):3622. doi: 10.3791/3622. J Vis Exp. 2012. PMID: 22349214 Free PMC article.
-
The avian embryo to study development of the cardiac conduction system.Differentiation. 2016 Apr-Jun;91(4-5):90-103. doi: 10.1016/j.diff.2016.01.006. Epub 2016 Feb 5. Differentiation. 2016. PMID: 26856662 Review.
Cited by
-
Effect of In Ovo Injection of L-Arginine in Different Chicken Embryonic Development Stages on Post-Hatchability, Immune Response, and Myo-D and Myogenin Proteins.Animals (Basel). 2019 Jun 14;9(6):357. doi: 10.3390/ani9060357. Animals (Basel). 2019. PMID: 31207968 Free PMC article.
-
Relatively Rare Populations of Invasive Cells Drive Progression of Heterogeneous Tumors.Cell Mol Bioeng. 2024 Jan 5;17(1):7-24. doi: 10.1007/s12195-023-00792-w. eCollection 2024 Feb. Cell Mol Bioeng. 2024. PMID: 38435793 Free PMC article.
-
Towards human organ generation using interspecies blastocyst complementation: Challenges and perspectives for therapy.Front Cell Dev Biol. 2023 Jan 19;11:1070560. doi: 10.3389/fcell.2023.1070560. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36743411 Free PMC article. Review.
-
Wounded embryonic corneas exhibit nonfibrotic regeneration and complete innervation.Invest Ophthalmol Vis Sci. 2013 Sep 24;54(9):6334-44. doi: 10.1167/iovs.13-12504. Invest Ophthalmol Vis Sci. 2013. PMID: 24003085 Free PMC article.
-
The Chicken as a Model Organism to Study Heart Development.Cold Spring Harb Perspect Biol. 2020 Aug 3;12(8):a037218. doi: 10.1101/cshperspect.a037218. Cold Spring Harb Perspect Biol. 2020. PMID: 31767650 Free PMC article. Review.
References
-
- Auerbach R, Kubai L, Knighton D, Folkman J. A simple procedure for the long-term cultivation of chicken embryos. Dev Biol. 1974;41:391–394. - PubMed
-
- del Barrio MG, Nieto MA. Overexpression of Snail family members highlights their ability to promote chick neural crest formation. Development. 2002;129:1583–1593. - PubMed
-
- Brown ST, Wang J, Groves AK. Dlx gene expression during chick inner ear development. J Comp Neurol. 2005;483:48–65. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

