Effect of spironolactone and amiloride on thiazolidinedione-induced fluid retention in South Indian patients with type 2 diabetes
- PMID: 23184569
- PMCID: PMC3562862
- DOI: 10.2215/CJN.06330612
Effect of spironolactone and amiloride on thiazolidinedione-induced fluid retention in South Indian patients with type 2 diabetes
Abstract
Background and objectives: Thiazolidinediones (pioglitazone and rosiglitazone) induce renal epithelial sodium channel (ENaC)-mediated sodium reabsorption, resulting in plasma volume (PV) expansion. Incidence and long-term management of fluid retention induced by thiazolidinediones remain unclear.
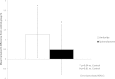
Design, setting, participants, & measurements: In a 4-week run-in period, rosiglitazone, 4 mg twice daily, was added to a background anti-diabetic therapy in 260 South Indian patients with type 2 diabetes mellitus. Patients with PV expansion (absolute reduction in hematocrit in run-in, ≥1.5 percentage points) entered a randomized, placebo-controlled study to evaluate effects of amiloride and spironolactone on attenuating rosiglitazone-induced fluid retention. Primary endpoint was change in hematocrit in each diuretic group versus placebo (control group).
Results: Of the 260 patients, 70% (n=180) had PV expansion. These 180 patients (70% male; mean age, 47.8 years [range, 30-80 years]) were randomly assigned to rosiglitazone, 4 mg twice daily, plus spironolactone, 50 mg once daily; rosiglitazone, 4 mg twice daily, plus amiloride, 10 mg once daily; or rosiglitazone, 4 mg twice daily, plus placebo for 24 weeks. Hematocrit continued to decrease significantly in control and spironolactone groups (mean absolute change, -1.2 [P=0.01] and -0.7 [P=0.02] percentage points, respectively), suggesting continued PV expansion. No change occurred with amiloride (mean change, 0.0 percentage points). Amiloride, but not spironolactone, was superior to control (mean hematocrit difference [95% confidence interval] relative to control, 1.27 [0.21-2.55] and 0.49 [-0.79-1.77] percentage points [P=0.04 and P=0.61], respectively).
Conclusions: Prevalence of rosiglitazone-induced fluid retention in South Indian patients with type 2 diabetes is high. Amiloride, a direct ENaC blocker, but not spironolactone, prevented protracted fluid retention in these patients.
Figures
References
-
- Yki-Jarvinen H: Thiazolidinediones. N Engl J Med 351:1106–1118, 2004 - PubMed
-
- Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Le Winter M, Porte D, Semenkovich CF, Smith S, Young LH, Kahn R: Thiazolidinedione use, fluid retention, and congestive heart failure: A consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care 27:256–263, 2004 - PubMed
-
- Nissen SE, Wolski K: Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356: 2457–2471 - PubMed
-
- Rosen CJ: Revisiting the rosiglitazone story—lessons learned. N Engl J Med 363:803–8066, 2010 - PubMed
-
- Karalliedde J, Viberti GC: Comment on: Boden et al. Combined use of rosiglitazone and fenofibrate in patients with type 2 diabetes: Prevention of fluid retention. Diabetes 56:e3; author reply e4, 2007 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

