Reducing HDAC6 ameliorates cognitive deficits in a mouse model for Alzheimer's disease
- PMID: 23184605
- PMCID: PMC3569653
- DOI: 10.1002/emmm.201201923
Reducing HDAC6 ameliorates cognitive deficits in a mouse model for Alzheimer's disease
Abstract
Histone deacetylases (HDACs) are currently being discussed as promising therapeutic targets to treat neurodegenerative diseases. However, the role of specific HDACs in cognition and neurodegeneration remains poorly understood. Here, we investigate the function of HDAC6, a class II member of the HDAC superfamily, in the adult mouse brain. We report that mice lacking HDAC6 are cognitively normal but reducing endogenous HDAC6 levels restores learning and memory and α-tubulin acetylation in a mouse model for Alzheimer's disease (AD). Our data suggest that this therapeutic effect is, at least in part, linked to the observation that loss of HDAC6 renders neurons resistant to amyloid-β-mediated impairment of mitochondrial trafficking. Thus, our study suggests that targeting HDAC6 could be a suitable strategy to ameliorate cognitive decline observed in AD.
Copyright © 2013 The Authors. Published by John Wiley and Sons, Ltd on behalf of EMBO.
Figures
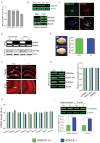
Quantitative real-time PCR showing normalized Hdac6 expression in different mouse brain regions. (n = 4, Student's t-test, PCx versus Cb: p = 0.0001, Hip versus Cb: p = 0.0107, Cx versus Cb: p = 0.0066).
Upper panel: Immunoblot analysis showing the HDAC6 protein levels in different brain regions. Lower panel: Immunoblot showing the predominant localization of HDAC6 to the cytoplasm.
Representative images showing cytoplasmic localization of viral-expressed HDAC6-GFP protein in primary hippocampal neurons (Scale bar: 10 µm).
PCR (upper panel) and immunoblot analysis (lower panel) confirming loss of Hdac6 mRNA and protein in the hippocampus and cortex of Hdac6−/− mice.
Representative brain images and brain mass in adult Hdac6−/− and wild type mice.
Representative images showing similar immunoreactivity of NeuN (scale bar: 100 µm) and SYP (scale bar: 50 µm) in Hdac6−/− and wild type mice.
Immunoblot (left) showing hippocampal histone acetylation in Hdac6−/− mice and wild type littermates along with densitometric quantification (right).
qPCR analysis of mRNA levels of other HDACs in Hdac6−/− mice and wild type littermates.
Quantitative immunoblot analysis showing elevated α-tubulin K40ac levels in the hippocampus and cortex of Hdac6−/− mice compared to wild type mice.

Representative images showing exploratory behaviour in the open field test.
Total distance covered during a 5 min open field exposure by Hdac6−/− and wild type mice (n = 10).
The time spent in the center versus periphery of the open field in Hdac6−/− and wild type mice (n = 10).
Motor function was analysed in the Rotarod test. Time spent on the rotating rod in Hdac6−/− and wild type mice (n = 10).
The total activity and the response to the electric foot shock in the contextual fear conditioning paradigm in Hdac6−/− and wild type mice (n = 10).
Contextual freezing behaviour assessed 24 h after the training in Hdac6−/− and wild type mice (n = 10).
Escape latency during the training phase of the Morris water maze in Hdac6−/− and wild type mice (n = 15).
Time spent in the target quadrant during the probe test in Hdac6−/− and wild type mice (n = 15). Values are mean ± SEM *p = 0.0384, ***p = 0.0001, analysed by Student's t-test. QT, Target quadrant.
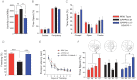
Total activity in the open field in wild type, APPPS1-21 and APPPS1-21-Hdac6−/− mice (n = 8, Student's t-test, p = 0.0289).
Time spent in the centre versus the periphery of the open field.
Time spent in the centre and periphery of the elevated plus-maze in wild type, APPPS1-21 and APPPS1-21-Hdac6−/− mice.
Freezing behaviour analysed 24 h after fear conditioning training in wild type (n = 9), APPPS1-21 (n = 11) and APPPS1-21-Hdac6−/− mice (n = 10), analysed by Student's t-test, p = 0.0005.
Escape latency during the Morris water maze training in wild type, APPPS1-21 and APPPS1-21-Hdac6−/− mice.
Target preference analysed in the probe test by comparing times spent by wild type, APPPS1-21 and APPPS1-21-Hdac6−/− mice in different quadrants on the water maze, analysed by Student's t-test, *p = 0.0036. The upper panel indicates representative swim paths during the probe test. Values are mean ± SEM.

Left panel: Representative confocal microscopy images showing immunoreactivity against Aβ in the hippocampus and cortex of APPPS1-21 (n = 6) and APPPS1-21-Hdac6−/− mice (n = 7). Scale bar: 100 µm. Right panel: Corresponding quantification of Aβ plaque load.
Representative immunoblot (upper) and quantitative analysis (lower) showing reduced levels of α-tubulin K40 acetylation in APPPS1-21 mice compared to wild type animals (n = 6, Student's t-test, p = 0.0024).
Representative immunoblot (upper) and quantitative analysis (lower) showing increased levels of HDAC6 in APPPS1-21 mice compared to wild type animals (n = 4, Student's t-test, p = 0.0212).
Representative immunoblot (upper) and corresponding quantification (lower) showing increased levels of α-tubulin K40ac in the hippocampus of APPPS1-21-Hdac6−/− compared to APPPS1-21 mice (n = 4, Student's t-test, p = 0.0007).
Quantitative analysis depicting the distribution of mitochondrial trafficking at a distinct speed in primary hippocampal neurons isolated from Hdac6−/− and wild type mice.
Percentage of motile mitochondria out of total mitochondria in the experiment described under (E).
Experimental design. Primary hippocampal neurons from Hdac6−/− and wild type littermates were treated with ADDL for 30 or 60 min and mitochondrial trafficking was analysed.
Upper panel: Representative time lapse images showing moving mitochondria in ADDL treated wild type and Hdac6−/− neurons before (0 min) and 30 or 60 min after treatment. Scale bar: 5 µm. Lower panel: Quantitative analysis shows ADDL-mediated impairment in mitochondrial trafficking in wild type (0 min vs. 30 or 60 min) but not in Hdac6−/− neurons (n = 6, Student's t-test, **p = 0.0053).
Left panel: Representative images showing Tom20 and tubulinK40ac immunoreactivity in the hippocampus of wild type (n = 8), APPPS1-21 (n = 10) and APPPS1-21_HDAC6−/− mice (n = 18). Dashed lines indicate areas used for quantification. Scale bar: 50 µm for low magnification images and 10 µm for Tom20 high magnification images (high-mag.). Right panel: Quantification of Tom20 immunoreactivity displayed as the ratio of intensity in soma to that in str. rad.
Similar articles
-
Tubastatin A/ACY-1215 improves cognition in Alzheimer's disease transgenic mice.J Alzheimers Dis. 2014;41(4):1193-1205. doi: 10.3233/JAD-140066. J Alzheimers Dis. 2014. PMID: 24844691
-
Increased acetylation of Peroxiredoxin1 by HDAC6 inhibition leads to recovery of Aβ-induced impaired axonal transport.Mol Neurodegener. 2017 Feb 28;12(1):23. doi: 10.1186/s13024-017-0164-1. Mol Neurodegener. 2017. PMID: 28241840 Free PMC article.
-
Hdac6 knock-out increases tubulin acetylation but does not modify disease progression in the R6/2 mouse model of Huntington's disease.PLoS One. 2011;6(6):e20696. doi: 10.1371/journal.pone.0020696. Epub 2011 Jun 3. PLoS One. 2011. PMID: 21677773 Free PMC article.
-
HDAC6 α-tubulin deacetylase: a potential therapeutic target in neurodegenerative diseases.J Neurol Sci. 2011 May 15;304(1-2):1-8. doi: 10.1016/j.jns.2011.02.017. Epub 2011 Mar 5. J Neurol Sci. 2011. PMID: 21377170 Review.
-
HDAC6 as a target for neurodegenerative diseases: what makes it different from the other HDACs?Mol Neurodegener. 2013 Jan 29;8:7. doi: 10.1186/1750-1326-8-7. Mol Neurodegener. 2013. PMID: 23356410 Free PMC article. Review.
Cited by
-
Clinical validation of the novel HDAC6 radiotracer [18F]EKZ-001 in the human brain.Eur J Nucl Med Mol Imaging. 2021 Feb;48(2):596-611. doi: 10.1007/s00259-020-04891-y. Epub 2020 Jul 8. Eur J Nucl Med Mol Imaging. 2021. PMID: 32638097 Free PMC article.
-
Neuronal epigenetics and the aging synapse.Front Cell Neurosci. 2015 May 27;9:208. doi: 10.3389/fncel.2015.00208. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26074775 Free PMC article. Review.
-
A Mitocentric View of Alzheimer's Disease.Mol Neurobiol. 2017 Oct;54(8):6046-6060. doi: 10.1007/s12035-016-0117-7. Epub 2016 Oct 1. Mol Neurobiol. 2017. PMID: 27696116 Review.
-
Synthesis and biological evaluation of novel quinolone derivatives dual targeting histone deacetylase and tubulin polymerization as antiproliferative agents.RSC Adv. 2018 May 4;8(30):16494-16502. doi: 10.1039/c8ra02578a. eCollection 2018 May 3. RSC Adv. 2018. PMID: 35540517 Free PMC article.
-
Histone-acetylation: a link between Alzheimer's disease and post-traumatic stress disorder?Front Neurosci. 2014 Jun 24;8:160. doi: 10.3389/fnins.2014.00160. eCollection 2014. Front Neurosci. 2014. PMID: 25009454 Free PMC article. Review.
References
-
- Bertram L, Tanzi RE. Thirty years of Alzheimer's disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci. 2008;9:768–778. - PubMed
-
- Butler DBJ, Michaelis ML, Karanian DA, Bahr BA. Microtubule-stabilizing agent prevents protein accumulation-induced loss of synaptic markers. Eur J Pharmacol. 2007;562:20–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

