Efficacy and safety of cytoreductive therapies in patients with essential thrombocythaemia aged >80 years: an interim analysis of the EXELS study
- PMID: 23184668
- PMCID: PMC3586170
- DOI: 10.1007/s40261-012-0042-0
Efficacy and safety of cytoreductive therapies in patients with essential thrombocythaemia aged >80 years: an interim analysis of the EXELS study
Abstract
Background: The median age of patients diagnosed with essential thrombocythaemia (ET) is 65-70 years but the management of very elderly patients (aged >80 years) with ET has not been well characterized.
Objective: This study aimed to document the treatment patterns of very elderly patients with ET in a multinational, real-world setting.
Study design: EXELS (Evaluation of Xagrid Efficacy and Long-term Safety) is a phase IV observational study, designed to monitor the efficacy and safety of cytoreductive therapies in clinical practice. In total, 3,598 high-risk patients with ET were recruited from May 2005 to April 2009, in 13 European countries. Data were collected at registration and every 6 months thereafter for 5 years. This analysis was performed on a data-cut taken approximately 2 years after the last patient was registered.
Patients: In total, 395 patients aged >80 years at registration into EXELS were included in the analysis; of these, 42.2 % had experienced a previous thrombohaemorrhagic event.
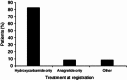
Results: At registration, the most frequently prescribed cytoreductive therapy for patients aged >80 years was hydroxycarbamide (HC), which accounted for 82.8 % of patients whereas anagrelide use was less frequent (8.6 %). Very elderly patients were more likely to be switched from anagrelide than from HC (47.1 vs. 17.4 %; 95 % confidence interval for difference in proportion 12.4-46.9; Chi-squared test p < 0.001). Median platelet count during treatment was ~430 × 10(9)/L. In patients aged >80 years, the main reason for switch was intolerance/side effects (34.1 %); 0/16 patients reported treatment with anagrelide was non-efficacious compared with 8/57 (14 %) patients receiving HC, and 7/16 (43.8 %) anagrelide patients switched because of intolerance versus 18/57 (31.6 %) patients receiving HC. At least one predefined clinical event (PDE) was experienced by 27.3 % of patients aged >80 years. The most common PDEs reported in the very elderly age group were death (non-PDE related; 11.1 %), other cardiovascular symptoms (5.8 %), haematological transformation (3.8 %), congestive heart failure (3.3 %), myocardial infarction and angina (2.8 %), and thromboembolic events (6.3 %).
Conclusion: Well-tolerated and effective cytoreductive therapy has been achieved in patients aged >80 years by following individual treatment modalities that appear in agreement with the recent European LeukemiaNet (ELN) guidelines.
Trial registration: ClinicalTrials.gov NCT00567502.
Figures


References
-
- Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haemopoietic and Lymphoid Tissues. 4. Lyon: IARC Press; 2008.
-
- Cortelazzo S, Viero P, Finazzi G, et al. Incidence and risk factors for thrombotic complications in a historical cohort of 100 patients with essential thrombocythemia. J Clin Oncol. 1990;8:556–562. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

