Stromal epigenetic dysregulation is sufficient to initiate mouse prostate cancer via paracrine Wnt signaling
- PMID: 23184966
- PMCID: PMC3528570
- DOI: 10.1073/pnas.1217982109
Stromal epigenetic dysregulation is sufficient to initiate mouse prostate cancer via paracrine Wnt signaling
Abstract
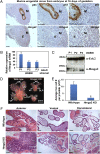
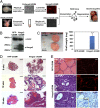
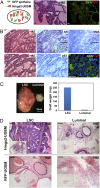
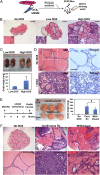
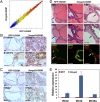
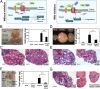
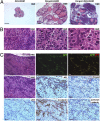
Carcinomas most often result from the stepwise acquisition of genetic alterations within the epithelial compartment. The surrounding stroma can also play an important role in cancer initiation and progression. Given the rare frequencies of genetic events identified in cancer-associated stroma, it is likely that epigenetic changes in the tumor microenvironment could contribute to its tumor-promoting activity. We use Hmga2 (High-mobility group AT-hook 2) an epigenetic regulator, to modify prostate stromal cells, and demonstrate that perturbation of the microenvironment by stromal-specific overexpression of this chromatin remodeling protein alone is sufficient to induce dramatic hyperplasia and multifocal prostatic intraepithelial neoplasia lesions from adjacent naïve epithelial cells. Importantly, we find that this effect is predominantly mediated by increased Wnt/β-catenin signaling. Enhancement of Hmga2-induced paracrine signaling by overexpression of androgen receptor in the stroma drives frank murine prostate adenocarcinoma in the adjacent epithelial tissues. Our findings provide compelling evidence for the critical contribution of epigenetic changes in stromal cells to multifocal tumorigenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. - PubMed
-
- Andreoiu M, Cheng L. Multifocal prostate cancer: Biologic, prognostic, and therapeutic implications. Hum Pathol. 2010;41(6):781–793. - PubMed
-
- Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6(5):963–968. - PubMed
-
- Bhowmick NA, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303(5659):848–851. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

