Direct three-dimensional visualization of membrane disruption by amyloid fibrils
- PMID: 23184970
- PMCID: PMC3528594
- DOI: 10.1073/pnas.1206325109
Direct three-dimensional visualization of membrane disruption by amyloid fibrils
Abstract
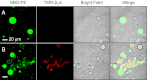
Protein misfolding and aggregation cause serious degenerative conditions such as Alzheimer's, Parkinson, and prion diseases. Damage to membranes is thought to be one of the mechanisms underlying cellular toxicity of a range of amyloid assemblies. Previous studies have indicated that amyloid fibrils can cause membrane leakage and elicit cellular damage, and these effects are enhanced by fragmentation of the fibrils. Here we report direct 3D visualization of membrane damage by specific interactions of a lipid bilayer with amyloid-like fibrils formed in vitro from β(2)-microglobulin (β(2)m). Using cryoelectron tomography, we demonstrate that fragmented β(2)m amyloid fibrils interact strongly with liposomes and cause distortions to the membranes. The normally spherical liposomes form pointed teardrop-like shapes with the fibril ends seen in proximity to the pointed regions on the membranes. Moreover, the tomograms indicated that the fibrils extract lipid from the membranes at these points of distortion by removal or blebbing of the outer membrane leaflet. Tiny (15-25 nm) vesicles, presumably formed from the extracted lipids, were observed to be decorating the fibrils. The findings highlight a potential role of fibrils, and particularly fibril ends, in amyloid pathology, and report a previously undescribed class of lipid-protein interactions in membrane remodelling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475(7356):324–332. - PubMed
-
- Pepys MB. Amyloidosis. Annu Rev Med. 2006;57:223–241. - PubMed
-
- Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–991. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

