pH-Triggered reversible morphological inversion of orthogonally-addressable poly(3-acrylamidophenylboronic acid)-block-poly(acrylamidoethylamine) micelles and their shell crosslinked nanoparticles
- PMID: 23185105
- PMCID: PMC3505036
- DOI: 10.1039/C2PY20324C
pH-Triggered reversible morphological inversion of orthogonally-addressable poly(3-acrylamidophenylboronic acid)-block-poly(acrylamidoethylamine) micelles and their shell crosslinked nanoparticles
Abstract
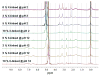
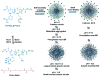
Functionally-responsive amphiphilic core-shell nanoscopic objects, capable of either complete or partial inversion processes, were produced by the supramolecular assembly of pH-responsive block copolymers, without or with covalent crosslinking of the shell layer, respectively. A new type of well-defined, dual-functionalized boronic acid- and amino-based diblock copolymer poly(3-acrylamidophenylboronic acid)(30)-block-poly(acrylamidoethylamine)(25) (PAPBA(30)-b-PAEA(25)) was synthesized by sequential reversible addition-fragmentation chain transfer (RAFT) polymerization and then assembled into cationic micelles in aqueous solution at pH 5.5. The micelles were further cross-linked throughout the shell domain comprised of poly(acrylamidoethylamine) by reaction with a bis-activated ester of 4,15-dioxo-8,11-dioxa-5,14-diazaoctadecane-1,18-dioic acid, upon increase of the pH to 7, to different cross-linking densities (2%, 5% and 10%), forming well-defined shell cross-linked nanoparticles (SCKs) with hydrodynamic diameters of ca. 50 nm. These smart micelles and SCKs presented switchable cationic, zwitterionic and anionic properties, and existed as stable nanoparticles with high positive surface charge at low pH (pH = 2, zeta potential ~ +40 mV) and strong negative surface charge at high pH (pH = 12, zeta potential ~ -35 mV). (1)H NMR spectroscopy, X-ray photoelectron spectroscopy (XPS), dynamic light scattering (DLS), transmission electron microscopy (TEM), atomic force microscopy (AFM), and zeta potential, were used to characterize the chemical compositions, particle sizes, morphologies and surface charges. Precipitation occurred near the isoelectric points (IEP) of the polymer/particle solutions, and the IEP values could be tuned by changing the shell cross-linking density. The block copolymer micelles were capable of full reversible morphological inversion as a function of pH, by orthogonal protonation of the PAEA and hydroxide association with the PAPBA units, whereas the SCKs underwent only reptation of the PAPBA chain segments through the crosslinked shell of PAEA as the pH was elevated. Further, these nanomaterials also showed D-glucose-responsive properties.
Figures






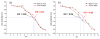
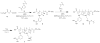
References
-
- Alarcon CDH, Pennadam S, Alexander C. Chemical Society Reviews. 2005;34:276–285. - PubMed
-
- Chen DY, Jiang M. Accounts of Chemical Research. 2005;38:494–502. - PubMed
-
- Dimitrov I, Trzebicka B, Muller AHE, Dworak A, Tsvetanov CB. Progress in Polymer Science. 2007;32:1275–1343.
-
- Gil ES, Hudson SM. Progress in Polymer Science. 2004;29:1173–1222.
-
- Stuart MAC, Huck WTS, Genzer J, Muller M, Ober C, Stamm M, Sukhorukov GB, Szleifer I, Tsukruk VV, Urban M, Winnik F, Zauscher S, Luzinov I, Minko S. Nature Materials. 2010;9:101–113. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
