Metformin and its clinical use: new insights for an old drug in clinical practice
- PMID: 23185203
- PMCID: PMC3506244
- DOI: 10.5114/aoms.2012.31622
Metformin and its clinical use: new insights for an old drug in clinical practice
Abstract
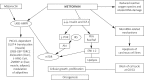
Metformin is generally recommended as first-line treatment in type 2 diabetes, especially in overweight patients, but in recent years new indications for its use have emerged. Metformin has been found to be safe and efficacious both as monotherapy and in combination with all oral antidiabetic agents and insulins. If metformin use during pregnancy and the lactation period is supported by few data, it could be indicated for women with polycystic ovary syndrome, since it could diminish circulating androgens and insulin resistance, thus ameliorating the ovulation rate. Metformin seems to reduce cancer risk, which appears to be increased in diabetics, and is a promising agent for oncoprevention and chemotherapy combinations. Moreover, metformin could find a place in the treatment of non-alcoholic fatty liver disease. Lactic acidosis could be decreased by avoiding metformin use in patients with hypovolemia, sepsis, renal impairment, hypoxic respiratory diseases and heart failure, in the preoperative period and before intravenous injection of contrast media.
Keywords: cancer; efficacy; metformin; safety; type 2 diabetes.
Figures
References
-
- Goldman-Levine JD. Beyond metformin: initiating combination therapy in patients with type 2 diabetes mellitus. Pharmacotherapy. 2011;31:44S–53S. - PubMed
-
- Nathan DM, Buse JB, Davidson MB, Management of hyperglycaemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy A Consensus Statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2006;49:1711–21. - PubMed
-
- Scarpello JHB. Optimal dosing strategies for modelling the clinical response to metformin in type 2 diabetes. Br J Diabetes Vasc Dis. 2001;1:28–36.
-
- Garber AJ, Duncan TG, Goodman AM, Mills DJ, Rohlf JL. Efficacy of metformin in type II diabetes: result of a double-blind, placebo-controlled, dose-response trial. Am J Med. 1997;103:491–7. - PubMed
-
- Fujioka K, Brazg RL, Raz I, et al. Efficacy, dose-response relationship and safety of once-daily extended release metformin (Glucophage XR) in type 2 diabetic patients with inadequate glycemic control despite prior treatment with diet and exercise: results from two double-blind, placebo-controlled studies. Diabetes Obes Metab. 2005;7:28–39. - PubMed
LinkOut - more resources
Full Text Sources
