Autoproteolysis and intramolecular dissociation of Yersinia YscU precedes secretion of its C-terminal polypeptide YscU(CC)
- PMID: 23185318
- PMCID: PMC3504009
- DOI: 10.1371/journal.pone.0049349
Autoproteolysis and intramolecular dissociation of Yersinia YscU precedes secretion of its C-terminal polypeptide YscU(CC)
Abstract
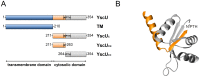
Type III secretion system mediated secretion and translocation of Yop-effector proteins across the eukaryotic target cell membrane by pathogenic Yersinia is highly organized and is dependent on a switching event from secretion of early structural substrates to late effector substrates (Yops). Substrate switching can be mimicked in vitro by modulating the calcium levels in the growth medium. YscU that is essential for regulation of this switch undergoes autoproteolysis at a conserved N↑PTH motif, resulting in a 10 kDa C-terminal polypeptide fragment denoted YscU(CC). Here we show that depletion of calcium induces intramolecular dissociation of YscU(CC) from YscU followed by secretion of the YscU(CC) polypeptide. Thus, YscU(CC) behaved in vivo as a Yop protein with respect to secretion properties. Further, destabilized yscU mutants displayed increased rates of dissociation of YscU(CC)in vitro resulting in enhanced Yop secretion in vivo at 30°C relative to the wild-type strain.These findings provide strong support to the relevance of YscU(CC) dissociation for Yop secretion. We propose that YscU(CC) orchestrates a block in the secretion channel that is eliminated by calcium depletion. Further, the striking homology between different members of the YscU/FlhB family suggests that this protein family possess regulatory functions also in other bacteria using comparable mechanisms.
Conflict of interest statement
Figures



Similar articles
-
YscU/FlhB of Yersinia pseudotuberculosis Harbors a C-terminal Type III Secretion Signal.J Biol Chem. 2015 Oct 23;290(43):26282-91. doi: 10.1074/jbc.M114.633677. Epub 2015 Sep 3. J Biol Chem. 2015. PMID: 26338709 Free PMC article.
-
Autoproteolysis of YscU of Yersinia pseudotuberculosis is important for regulation of expression and secretion of Yop proteins.J Bacteriol. 2009 Jul;191(13):4259-67. doi: 10.1128/JB.01730-08. Epub 2009 Apr 24. J Bacteriol. 2009. PMID: 19395493 Free PMC article.
-
Proteolytic cleavage of the FlhB homologue YscU of Yersinia pseudotuberculosis is essential for bacterial survival but not for type III secretion.J Bacteriol. 2002 Aug;184(16):4500-9. doi: 10.1128/JB.184.16.4500-4509.2002. J Bacteriol. 2002. PMID: 12142420 Free PMC article.
-
Links between type III secretion and extracytoplasmic stress responses in Yersinia.Front Cell Infect Microbiol. 2012 Oct 10;2:125. doi: 10.3389/fcimb.2012.00125. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 23087910 Free PMC article. Review.
-
The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells.Mol Microbiol. 1997 Mar;23(5):861-7. doi: 10.1046/j.1365-2958.1997.2731623.x. Mol Microbiol. 1997. PMID: 9076724 Review.
Cited by
-
Negatively charged lipid membranes promote a disorder-order transition in the Yersinia YscU protein.Biophys J. 2014 Oct 21;107(8):1950-1961. doi: 10.1016/j.bpj.2014.09.005. Biophys J. 2014. PMID: 25418176 Free PMC article.
-
Transcriptomic profiling of Yersinia pseudotuberculosis reveals reprogramming of the Crp regulon by temperature and uncovers Crp as a master regulator of small RNAs.PLoS Genet. 2015 Mar 27;11(3):e1005087. doi: 10.1371/journal.pgen.1005087. eCollection 2015 Mar. PLoS Genet. 2015. PMID: 25816203 Free PMC article.
-
Genetically engineered frameshifted YopN-TyeA chimeras influence type III secretion system function in Yersinia pseudotuberculosis.PLoS One. 2013 Oct 3;8(10):e77767. doi: 10.1371/journal.pone.0077767. eCollection 2013. PLoS One. 2013. PMID: 24098594 Free PMC article.
-
YscU/FlhB of Yersinia pseudotuberculosis Harbors a C-terminal Type III Secretion Signal.J Biol Chem. 2015 Oct 23;290(43):26282-91. doi: 10.1074/jbc.M114.633677. Epub 2015 Sep 3. J Biol Chem. 2015. PMID: 26338709 Free PMC article.
-
Bacterial type III secretion systems: a complex device for the delivery of bacterial effector proteins into eukaryotic host cells.FEMS Microbiol Lett. 2018 Oct 1;365(19):fny201. doi: 10.1093/femsle/fny201. FEMS Microbiol Lett. 2018. PMID: 30107569 Free PMC article. Review.
References
-
- Hills GM, Spurr ED (1952) The effect of temperature on the nutritional requirements of Pasteurella pestis. J Gen Microbiol 6: 64–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

