Expression of ESE-3 isoforms in immunogenic and tolerogenic human monocyte-derived dendritic cells
- PMID: 23185370
- PMCID: PMC3501485
- DOI: 10.1371/journal.pone.0049577
Expression of ESE-3 isoforms in immunogenic and tolerogenic human monocyte-derived dendritic cells
Abstract
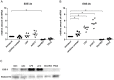
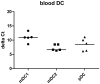
Dendritic cells (DC) are the only hematopoietic cells expressing the epithelial specific Ets transcription factor ESE-3. Here we analyzed presence and quantity of isoforms ESE-3a, ESE-3b and ESE-3j in various immunogenic and tolerogenic human monocyte-derived DC (moDC) and blood DC populations using quantitative real time PCR and immunoblot analyses. ESE-3a and ESE-3b were detectable in all moDC populations with ESE-3b being the main transcript. ESE-3b expression was upregulated in immunogenic moDC and downregulated in tolerogenic moDC compared to immature moDC. ESE-3a had similar transcript levels in immature and immunogenic moDC and had very low levels in tolerogenic moDC. In blood DC populations only splice variant ESE-3b was detectable. ESE-3j was not detectable in any of the DC populations. These findings suggest that ESE-3b is the functionally most important ESE-3 isoform in DC.
Conflict of interest statement
Figures


Similar articles
-
Activation of peroxisome proliferator-activated receptor gamma leads to upregulation of ESE-3 expression in human monocyte-derived dendritic cells.Scand J Immunol. 2014 Jan;79(1):20-6. doi: 10.1111/sji.12126. Scand J Immunol. 2014. PMID: 24219556
-
The effect of age on foal monocyte-derived dendritic cell (MoDC) maturation and function after exposure to killed bacteria.Vet Immunol Immunopathol. 2019 Apr;210:38-45. doi: 10.1016/j.vetimm.2018.11.020. Epub 2019 Mar 9. Vet Immunol Immunopathol. 2019. PMID: 30947978
-
Phenotype and functional analysis of human monocyte-derived dendritic cells loaded with biodegradable poly(lactide-co-glycolide) microspheres for immunotherapy.J Immunol Methods. 2004 Apr;287(1-2):109-24. doi: 10.1016/j.jim.2004.01.010. J Immunol Methods. 2004. PMID: 15099760
-
CD16+ and CD16- human blood monocyte subsets differentiate in vitro to dendritic cells with different abilities to stimulate CD4+ T cells.Int Immunol. 2001 Dec;13(12):1571-81. doi: 10.1093/intimm/13.12.1571. Int Immunol. 2001. PMID: 11717198
-
Classical DC2 subsets and monocyte-derived DC: Delineating the developmental and functional relationship.Eur J Immunol. 2023 Mar;53(3):e2149548. doi: 10.1002/eji.202149548. Epub 2023 Feb 5. Eur J Immunol. 2023. PMID: 36642930 Review.
Cited by
-
Increased expression of EHF contributes to thyroid tumorigenesis through transcriptionally regulating HER2 and HER3.Oncotarget. 2016 Sep 6;7(36):57978-57990. doi: 10.18632/oncotarget.11154. Oncotarget. 2016. PMID: 27517321 Free PMC article.
-
Abnormal Localization and Tumor Suppressor Function of Epithelial Tissue-Specific Transcription Factor ESE3 in Esophageal Squamous Cell Carcinoma.PLoS One. 2015 May 7;10(5):e0126319. doi: 10.1371/journal.pone.0126319. eCollection 2015. PLoS One. 2015. PMID: 25950810 Free PMC article.
-
Increased expression of EHF via gene amplification contributes to the activation of HER family signaling and associates with poor survival in gastric cancer.Cell Death Dis. 2016 Oct 27;7(10):e2442. doi: 10.1038/cddis.2016.346. Cell Death Dis. 2016. PMID: 27787520 Free PMC article.
-
ELF3, ELF5, EHF and SPDEF Transcription Factors in Tissue Homeostasis and Cancer.Molecules. 2018 Aug 30;23(9):2191. doi: 10.3390/molecules23092191. Molecules. 2018. PMID: 30200227 Free PMC article. Review.
References
-
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, et al. (2000) Immunobiology of dendritic cells. Annu Rev Immunol 18: 767–811. - PubMed
-
- Steinman RM, Hawiger D, Nussenzweig MC (2003) Tolerogenic dendritic cells. Annu Rev Immunol 21: 685–711. - PubMed
-
- Banchereau J, Pascual V, Palucka AK (2004) Autoimmunity through cytokine-induced dendritic cell activation. Immunity 20: 539–550. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

