Dysferlin-peptides reallocate mutated dysferlin thereby restoring function
- PMID: 23185377
- PMCID: PMC3502493
- DOI: 10.1371/journal.pone.0049603
Dysferlin-peptides reallocate mutated dysferlin thereby restoring function
Abstract
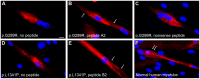
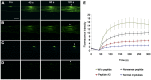
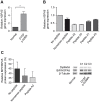
Mutations in the dysferlin gene cause the most frequent adult-onset limb girdle muscular dystrophy, LGMD2B. There is no therapy. Dysferlin is a membrane protein comprised of seven, beta-sheet enriched, C2 domains and is involved in Ca(2+)dependent sarcolemmal repair after minute wounding. On the protein level, point mutations in DYSF lead to misfolding, aggregation within the endoplasmic reticulum, and amyloidogenesis. We aimed to restore functionality by relocating mutant dysferlin. Therefore, we designed short peptides derived from dysferlin itself and labeled them to the cell penetrating peptide TAT. By tracking fluorescently labeled short peptides we show that these dysferlin-peptides localize in the endoplasmic reticulum. There, they are capable of reducing unfolded protein response stress. We demonstrate that the mutant dysferlin regains function in membrane repair in primary human myotubes derived from patients' myoblasts by the laser wounding assay and a novel technique to investigate membrane repair: the interventional atomic force microscopy. Mutant dysferlin abuts to the sarcolemma after peptide treatment. The peptide-mediated approach has not been taken before in the field of muscular dystrophies. Our results could redirect treatment efforts for this condition.
Conflict of interest statement
Figures



Similar articles
-
Dysferlin interacts with affixin (beta-parvin) at the sarcolemma.J Neuropathol Exp Neurol. 2005 Apr;64(4):334-40. doi: 10.1093/jnen/64.4.334. J Neuropathol Exp Neurol. 2005. PMID: 15835269
-
Novel sequence variants in dysferlin-deficient muscular dystrophy leading to mRNA decay and possible C2-domain misfolding.Hum Mutat. 2006 Jun;27(6):599-600. doi: 10.1002/humu.9424. Hum Mutat. 2006. PMID: 16705711
-
Proteasome inhibitors increase missense mutated dysferlin in patients with muscular dystrophy.Sci Transl Med. 2014 Aug 20;6(250):250ra112. doi: 10.1126/scitranslmed.3009612. Epub 2014 Aug 20. Sci Transl Med. 2014. Retraction in: Sci Transl Med. 2017 Jun 7;9(393):eaan8388. doi: 10.1126/scitranslmed.aan8388. PMID: 25143362 Retracted. Clinical Trial.
-
Translational research and therapeutic perspectives in dysferlinopathies.Mol Med. 2011 Sep-Oct;17(9-10):875-82. doi: 10.2119/molmed.2011.00084. Epub 2011 May 6. Mol Med. 2011. PMID: 21556485 Free PMC article. Review.
-
Dysferlin function in skeletal muscle: Possible pathological mechanisms and therapeutical targets in dysferlinopathies.Exp Neurol. 2016 Sep;283(Pt A):246-54. doi: 10.1016/j.expneurol.2016.06.026. Epub 2016 Jun 25. Exp Neurol. 2016. PMID: 27349407 Review.
Cited by
-
Inhibition of inflammation with celastrol fails to improve muscle function in dysferlin-deficient A/J mice.J Neurol Sci. 2015 Sep 15;356(1-2):157-62. doi: 10.1016/j.jns.2015.06.042. Epub 2015 Jun 24. J Neurol Sci. 2015. PMID: 26119397 Free PMC article.
-
Brain of miyoshi myopathy/dysferlinopathy patients presents with structural and metabolic anomalies.Sci Rep. 2024 Aug 20;14(1):19267. doi: 10.1038/s41598-024-69966-4. Sci Rep. 2024. PMID: 39164335 Free PMC article.
-
Full-length Dysferlin Transfer by the Hyperactive Sleeping Beauty Transposase Restores Dysferlin-deficient Muscle.Mol Ther Nucleic Acids. 2016 Jan 19;5(1):e277. doi: 10.1038/mtna.2015.52. Mol Ther Nucleic Acids. 2016. PMID: 26784637 Free PMC article.
-
4-Phenylbutyrate restores localization and membrane repair to human dysferlin mutations.iScience. 2021 Dec 20;25(1):103667. doi: 10.1016/j.isci.2021.103667. eCollection 2022 Jan 21. iScience. 2021. PMID: 35028538 Free PMC article.
-
Treatment with Recombinant Human MG53 Protein Increases Membrane Integrity in a Mouse Model of Limb Girdle Muscular Dystrophy 2B.Mol Ther. 2017 Oct 4;25(10):2360-2371. doi: 10.1016/j.ymthe.2017.06.025. Epub 2017 Jul 3. Mol Ther. 2017. PMID: 28750735 Free PMC article.
References
-
- Anderson LV, Davison K, Moss JA, Young C, Cullen MJ, et al. (1999) Dysferlin is a plasma membrane protein and is expressed early in human development. Hum Mol Genet 8: 855–861. - PubMed
-
- Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, et al. (2003) Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature 423: 168–172. - PubMed
-
- Bashir R, Britton S, Strachan T, Keers S, Vafiadaki E, et al. (1998) A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat Genet 20: 37–42. - PubMed
-
- Liu J, Aoki M, Illa I, Wu C, Fardeau M, et al. (1998) Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat Genet 20: 31–36. - PubMed
-
- Krahn M, Beroud C, Labelle V, Nguyen K, Bernard R, et al. (2009) Analysis of the DYSF mutational spectrum in a large cohort of patients. Hum Mutat 30: E345–375. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

