Lipocalin 2 regulates inflammation during pulmonary mycobacterial infections
- PMID: 23185529
- PMCID: PMC3502292
- DOI: 10.1371/journal.pone.0050052
Lipocalin 2 regulates inflammation during pulmonary mycobacterial infections
Abstract
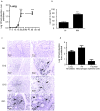
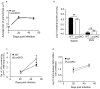
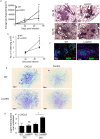
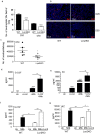
Pulmonary tuberculosis (TB), caused by the intracellular bacteria Mycobacterium tuberculosis, is a worldwide disease that continues to kill more than 1.5 million people every year worldwide. The accumulation of lymphocytes mediates the formation of the tubercle granuloma in the lung and is crucial for host protection against M.tuberculosis infection. However, paradoxically the tubercle granuloma is also the basis for the immunopathology associated with the disease and very little is known about the regulatory mechanisms that constrain the inflammation associated with the granulomas. Lipocalin 2 (Lcn2) is a member of the lipocalin family of proteins and binds to bacterial siderophores thereby sequestering iron required for bacterial growth. Thus far, it is not known whether Lcn2 plays a role in the inflammatory response to mycobacterial pulmonary infections. In the present study, using models of acute and chronic mycobacterial pulmonary infections, we reveal a novel role for Lcn2 in constraining T cell lymphocytic accumulation and inflammation by inhibiting inflammatory chemokines, such as CXCL9. In contrast, Lcn2 promotes neutrophil recruitment during mycobacterial pulmonary infection, by inducing G-CSF and KC in alveolar macrophages. Importantly, despite a common role for Lcn2 in regulating chemokines during mycobacterial pulmonary infections, Lcn2 deficient mice are more susceptible to acute M.bovis BCG, but not low dose M.tuberculosis pulmonary infection.
Conflict of interest statement
Figures
Similar articles
-
Lipocalin 2-dependent inhibition of mycobacterial growth in alveolar epithelium.J Immunol. 2008 Dec 15;181(12):8521-7. doi: 10.4049/jimmunol.181.12.8521. J Immunol. 2008. PMID: 19050270
-
The pivotal role played by lipocalin-2 in chronic inflammatory pain.Exp Neurol. 2014 Apr;254:41-53. doi: 10.1016/j.expneurol.2014.01.009. Epub 2014 Jan 17. Exp Neurol. 2014. PMID: 24440229
-
Lipocalin 2 deactivates macrophages and worsens pneumococcal pneumonia outcomes.J Clin Invest. 2013 Aug;123(8):3363-72. doi: 10.1172/JCI67911. Epub 2013 Jul 1. J Clin Invest. 2013. PMID: 23863624 Free PMC article.
-
The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer.Biochim Biophys Acta. 2012 Aug;1826(1):129-69. doi: 10.1016/j.bbcan.2012.03.008. Epub 2012 Mar 31. Biochim Biophys Acta. 2012. PMID: 22513004 Free PMC article. Review.
-
Lipocalin 2 in cancer: when good immunity goes bad.Cancer Lett. 2012 Mar 28;316(2):132-8. doi: 10.1016/j.canlet.2011.11.002. Epub 2011 Nov 7. Cancer Lett. 2012. PMID: 22075378 Review.
Cited by
-
Long-term evolution of the epithelial cell secretome in preclinical 3D models of the human bronchial epithelium.Sci Rep. 2021 Mar 23;11(1):6621. doi: 10.1038/s41598-021-86037-0. Sci Rep. 2021. PMID: 33758289 Free PMC article.
-
The mRNA expression of visfatin and lipocalin-2 in peripheral blood mononuclear cells from patients with pulmonary tuberculosis.J Clin Lab Anal. 2020 Nov;34(11):e23476. doi: 10.1002/jcla.23476. Epub 2020 Jul 18. J Clin Lab Anal. 2020. PMID: 32681594 Free PMC article.
-
Airway epithelial cells mount an early response to mycobacterial infection.Front Cell Infect Microbiol. 2023 Sep 26;13:1253037. doi: 10.3389/fcimb.2023.1253037. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37822359 Free PMC article.
-
Lipocalin-2 is an essential component of the innate immune response to Acinetobacter baumannii infection.PLoS Pathog. 2022 Sep 2;18(9):e1010809. doi: 10.1371/journal.ppat.1010809. eCollection 2022 Sep. PLoS Pathog. 2022. PMID: 36054235 Free PMC article.
-
IRF3 inhibits IFN-γ-mediated restriction of intracellular pathogens in macrophages independently of IFNAR.J Leukoc Biol. 2022 Aug;112(2):257-271. doi: 10.1002/JLB.3A0218-069RR. Epub 2021 Nov 26. J Leukoc Biol. 2022. PMID: 34826345 Free PMC article.
References
-
- Seiler P, Aichele P, Bandermann S, Hauser AE, Lu B, et al. (2003) Early granuloma formation after aerosol Mycobacterium tuberculosis infection is regulated by neutrophils via CXCR3-signaling chemokines. Eur J Immunol 33: 2676–2686. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

