Protein tyrosine phosphatase µ (PTP µ or PTPRM), a negative regulator of proliferation and invasion of breast cancer cells, is associated with disease prognosis
- PMID: 23185569
- PMCID: PMC3502354
- DOI: 10.1371/journal.pone.0050183
Protein tyrosine phosphatase µ (PTP µ or PTPRM), a negative regulator of proliferation and invasion of breast cancer cells, is associated with disease prognosis
Abstract
Background: PTPRM has been shown to exhibit homophilic binding and confer cell-cell adhesion in cells including epithelial and cancer cells. The present study investigated the expression of PTPRM in breast cancer and the biological impact of PTPRM on breast cancer cells.
Design: Expression of PTPRM protein and gene transcript was examined in a cohort of breast cancer patients. Knockdown of PTPRM in breast cancer cells was performed using a specific anti-PTPRM transgene. The impact of PTPRM knockdown on breast cancer was evaluated using in vitro cell models.
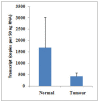
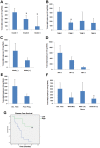
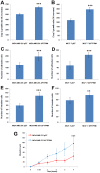
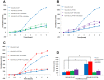
Results: A significant decrease of PTPRM transcripts was seen in poorly differentiated and moderately differentiated tumours compared with well differentiated tumours. Patients with lower expression of PTPRM had shorter survival compared with those which had a higher level of PTPRM expression. Knockdown of PTPRM increased proliferation, adhesion, invasion and migration of breast cancer cells. Furthermore, knockdown of PTPRM in MDA-MB-231 cells resulted in increased cell migration and invasion via regulation of the tyrosine phosphorylation of ERK and JNK.
Conclusions: Decreased expression of PTPRM in breast cancer is correlated with poor prognosis and inversely correlated with disease free survival. PTPRM coordinated cell migration and invasion through the regulation of tyrosine phosphorylation of ERK and JNK.
Conflict of interest statement
Figures



Similar articles
-
Protein tyrosine phosphatase kappa (PTPRK) is a negative regulator of adhesion and invasion of breast cancer cells, and associates with poor prognosis of breast cancer.J Cancer Res Clin Oncol. 2013 Jul;139(7):1129-39. doi: 10.1007/s00432-013-1421-5. Epub 2013 Apr 4. J Cancer Res Clin Oncol. 2013. PMID: 23552869 Free PMC article.
-
High expression of PTPRM predicts poor prognosis and promotes tumor growth and lymph node metastasis in cervical cancer.Cell Death Dis. 2020 Aug 11;11(8):687. doi: 10.1038/s41419-020-02826-x. Cell Death Dis. 2020. PMID: 32826853 Free PMC article.
-
The expression of the von Hippel-Lindau gene product and its impact on invasiveness of human breast cancer cells.Int J Mol Med. 2007 Oct;20(4):605-11. Int J Mol Med. 2007. PMID: 17786294
-
Regulation of development and cancer by the R2B subfamily of RPTPs and the implications of proteolysis.Semin Cell Dev Biol. 2015 Jan;37:108-18. doi: 10.1016/j.semcdb.2014.09.004. Epub 2014 Sep 16. Semin Cell Dev Biol. 2015. PMID: 25223585 Free PMC article. Review.
-
Molecular basis of invasion in breast cancer.Cell Mol Life Sci. 2007 Dec;64(24):3201-18. doi: 10.1007/s00018-007-7388-0. Cell Mol Life Sci. 2007. PMID: 17957337 Free PMC article. Review.
Cited by
-
Gene Profiling of a 3D Psoriatic Skin Model Enriched in T Cells: Downregulation of PTPRM Promotes Keratinocyte Proliferation through Excessive ERK1/2 Signaling.Cells. 2022 Sep 16;11(18):2904. doi: 10.3390/cells11182904. Cells. 2022. PMID: 36139479 Free PMC article.
-
Role of protein tyrosine phosphatase receptor type M in epithelial ovarian cancer progression.J Ovarian Res. 2023 Jul 4;16(1):131. doi: 10.1186/s13048-023-01220-3. J Ovarian Res. 2023. PMID: 37403117 Free PMC article.
-
Expression array analysis of the hepatocyte growth factor invasive program.Clin Exp Metastasis. 2015 Oct;32(7):659-76. doi: 10.1007/s10585-015-9735-0. Epub 2015 Aug 1. Clin Exp Metastasis. 2015. PMID: 26231668 Free PMC article.
-
Molecular Landscape of Endometrial Stromal Tumors.JCO Precis Oncol. 2025 May;9:e2400779. doi: 10.1200/PO-24-00779. Epub 2025 May 22. JCO Precis Oncol. 2025. PMID: 40403211 Free PMC article.
-
Loss of PTPRM associates with the pathogenic development of colorectal adenoma-carcinoma sequence.Sci Rep. 2015 Apr 24;5:9633. doi: 10.1038/srep09633. Sci Rep. 2015. PMID: 25910225 Free PMC article.
References
-
- Halle M, Tremblay ML, Meng TC (2007) Protein tyrosine phosphatases: emerging regulators of apoptosis. Cell Cycle 6: 2773–2781. - PubMed
-
- Angers-Loustau A, Cote JF, Tremblay ML (1999) Roles of protein tyrosine phosphatases in cell migration and adhesion. Biochem Cell Biol 77: 493–505. - PubMed
-
- Stoker AW (2005) Protein tyrosine phosphatases and signalling. J Endocrinol 185: 19–33. - PubMed
-
- Schiller KR, Mauro LJ (2005) Tyrosine phosphatases as regulators of skeletal development and metabolism. J Cell Biochem 96: 262–277. - PubMed
-
- Cully M, You H, Levine AJ, Mak TW (2006) Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer 6: 184–192. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

