Regulation of the formyl peptide receptor 1 (FPR1) gene in primary human macrophages
- PMID: 23185575
- PMCID: PMC3503994
- DOI: 10.1371/journal.pone.0050195
Regulation of the formyl peptide receptor 1 (FPR1) gene in primary human macrophages
Abstract
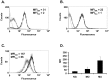
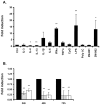
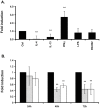
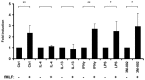
The formyl peptide receptor 1 (FPR1) is mainly expressed by mammalian phagocytic leukocytes and plays a role in chemotaxis, killing of microorganisms through phagocytosis, and the generation of reactive oxygen species. A large number of ligands have been identified triggering FPR1 including formylated and non-formylated peptides of microbial and endogenous origin. While the expression of FPR1 in neutrophils has been investigated intensively, knowledge on the regulation of FPR1 expression in polarized macrophages is lacking. In this study we show that primary human neutrophils, monocytes and resting macrophages do express the receptor on their cell surface. Polarization of macrophages with IFNγ, LPS and with the TLR8 ligand 3M-002 further increases FPR1 mRNA levels but does not consistently increase protein expression or chemotaxis towards the FPR1 ligand fMLF. In contrast, polarization of primary human macrophages with IL-4 and IL-13 leading to the alternative activated macrophages, reduces FPR1 cell surface expression and abolishes chemotaxis towards fMLF. These results show that M2 macrophages will not react to triggering of FPR1, limiting the role for FPR1 to chemotaxis and superoxide production of resting and pro-inflammatory M1 macrophages.
Conflict of interest statement
Figures
Similar articles
-
Identification of C-terminal phosphorylation sites of N-formyl peptide receptor-1 (FPR1) in human blood neutrophils.J Biol Chem. 2013 Sep 20;288(38):27042-27058. doi: 10.1074/jbc.M113.484113. Epub 2013 Jul 19. J Biol Chem. 2013. PMID: 23873933 Free PMC article.
-
The formyl peptide fMLF primes platelet activation and augments thrombus formation.J Thromb Haemost. 2019 Jul;17(7):1120-1133. doi: 10.1111/jth.14466. Epub 2019 May 24. J Thromb Haemost. 2019. PMID: 31033193 Free PMC article.
-
Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1.J Immunol. 2013 Jun 15;190(12):6511-9. doi: 10.4049/jimmunol.1202215. Epub 2013 May 13. J Immunol. 2013. PMID: 23670191
-
FPR1: A critical gatekeeper of the heart and brain.Pharmacol Res. 2024 Apr;202:107125. doi: 10.1016/j.phrs.2024.107125. Epub 2024 Mar 2. Pharmacol Res. 2024. PMID: 38438091 Review.
-
Effects of synthetic peptides on the inflammatory response and their therapeutic potential.Mini Rev Med Chem. 2013 Apr;13(4):553-64. doi: 10.2174/1389557511313040008. Mini Rev Med Chem. 2013. PMID: 22512576 Review.
Cited by
-
The role of formylated peptides and formyl peptide receptor 1 in governing neutrophil function during acute inflammation.Am J Pathol. 2015 May;185(5):1172-84. doi: 10.1016/j.ajpath.2015.01.020. Epub 2015 Mar 17. Am J Pathol. 2015. PMID: 25791526 Free PMC article. Review.
-
FAM19A4 is a novel cytokine ligand of formyl peptide receptor 1 (FPR1) and is able to promote the migration and phagocytosis of macrophages.Cell Mol Immunol. 2015 Sep;12(5):615-24. doi: 10.1038/cmi.2014.61. Epub 2014 Aug 11. Cell Mol Immunol. 2015. PMID: 25109685 Free PMC article.
-
Neutrophil Extracellular Trap Formation Potential Correlates with Lung Disease Severity in COVID-19 Patients.Inflammation. 2022 Apr;45(2):800-811. doi: 10.1007/s10753-021-01585-x. Epub 2021 Oct 31. Inflammation. 2022. PMID: 34718927 Free PMC article.
-
A functional genomics predictive network model identifies regulators of inflammatory bowel disease.Nat Genet. 2017 Oct;49(10):1437-1449. doi: 10.1038/ng.3947. Epub 2017 Sep 11. Nat Genet. 2017. PMID: 28892060 Free PMC article.
-
PET imaging detection of macrophages with a formyl peptide receptor antagonist.Nucl Med Biol. 2015 Apr;42(4):381-6. doi: 10.1016/j.nucmedbio.2014.12.001. Epub 2014 Dec 6. Nucl Med Biol. 2015. PMID: 25532700 Free PMC article.
References
-
- Fu H, Karlsson J, Bylund J, Movitz C, Karlsson A, et al. (2006) Ligand recognition and activation of formyl peptide receptors in neutrophils. J Leukoc Biol 79: 247–256. - PubMed
-
- Neptune ER, Iiri T, Bourne HR (1999) Galphai is not required for chemotaxis mediated by Gi-coupled receptors. J Biol Chem 274: 2824–2828. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

