Glucocorticoids regulation of FosB/ΔFosB expression induced by chronic opiate exposure in the brain stress system
- PMID: 23185589
- PMCID: PMC3503985
- DOI: 10.1371/journal.pone.0050264
Glucocorticoids regulation of FosB/ΔFosB expression induced by chronic opiate exposure in the brain stress system
Abstract
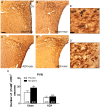
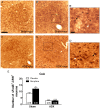
Chronic use of drugs of abuse profoundly alters stress-responsive system. Repeated exposure to morphine leads to accumulation of the transcription factor ΔFosB, particularly in brain areas associated with reward and stress. The persistent effects of ΔFosB on target genes may play an important role in the plasticity induced by drugs of abuse. Recent evidence suggests that stress-related hormones (e.g., glucocorticoids, GC) may induce adaptations in the brain stress system that is likely to involve alteration in gene expression and transcription factors. This study examined the role of GC in regulation of FosB/ΔFosB in both hypothalamic and extrahypothalamic brain stress systems during morphine dependence. For that, expression of FosB/ΔFosB was measured in control (sham-operated) and adrenalectomized (ADX) rats that were made opiate dependent after ten days of morphine treatment. In sham-operated rats, FosB/ΔFosB was induced after chronic morphine administration in all the brain stress areas investigated: nucleus accumbens(shell) (NAc), bed nucleus of the stria terminalis (BNST), central amygdala (CeA), hypothalamic paraventricular nucleus (PVN) and nucleus of the solitary tract noradrenergic cell group (NTS-A(2)). Adrenalectomy attenuated the increased production of FosB/ΔFosB observed after chronic morphine exposure in NAc, CeA, and NTS. Furthermore, ADX decreased expression of FosB/ΔFosB within CRH-positive neurons of the BNST, PVN and CeA. Similar results were obtained in NTS-A(2) TH-positive neurons and NAc pro-dynorphin-positive neurons. These data suggest that neuroadaptation (estimated as accumulation of FosB/ΔFosB) to opiates in brain areas associated with stress is modulated by GC, supporting the evidence of a link between brain stress hormones and addiction.
Conflict of interest statement
Figures





Similar articles
-
Induction of FosB/DeltaFosB in the brain stress system-related structures during morphine dependence and withdrawal.J Neurochem. 2010 Jul;114(2):475-87. doi: 10.1111/j.1471-4159.2010.06765.x. Epub 2010 Apr 23. J Neurochem. 2010. PMID: 20438612
-
Glucocorticoid dependency of surgical stress-induced FosB/DeltaFosB expression in the paraventricular and supraoptic nuclei of the rat hypothalamus.J Neuroendocrinol. 2009 Oct;21(10):822-31. doi: 10.1111/j.1365-2826.2009.01902.x. Epub 2009 Jul 21. J Neuroendocrinol. 2009. PMID: 19686449
-
Clozapine impact on FosB/ΔFosB expression in stress preconditioned rats: response to a novel stressor.Endocr Regul. 2019 Apr 1;53(2):83-92. doi: 10.2478/enr-2019-0010. Endocr Regul. 2019. PMID: 31517626
-
Reflections on: "A general role for adaptations in G-Proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function".Brain Res. 2016 Aug 15;1645:71-4. doi: 10.1016/j.brainres.2015.12.039. Epub 2015 Dec 29. Brain Res. 2016. PMID: 26740398 Free PMC article. Review.
-
∆FosB: a transcriptional regulator of stress and antidepressant responses.Eur J Pharmacol. 2015 Apr 15;753:66-72. doi: 10.1016/j.ejphar.2014.10.034. Epub 2014 Nov 7. Eur J Pharmacol. 2015. PMID: 25446562 Free PMC article. Review.
Cited by
-
Acute and chronic stress differentially regulate cyclin-dependent kinase 5 in mouse brain: implications to glucocorticoid actions and major depression.Transl Psychiatry. 2015 Jun 9;5(6):e578. doi: 10.1038/tp.2015.72. Transl Psychiatry. 2015. PMID: 26057048 Free PMC article.
-
Regulation of Pleiotrophin, Midkine, Receptor Protein Tyrosine Phosphatase β/ζ, and Their Intracellular Signaling Cascades in the Nucleus Accumbens During Opiate Administration.Int J Neuropsychopharmacol. 2015 Jul 11;19(1):pyv077. doi: 10.1093/ijnp/pyv077. Int J Neuropsychopharmacol. 2015. PMID: 26164717 Free PMC article.
-
Sex-Specific Timelines for Adaptations of Prefrontal Parvalbumin Neurons in Response to Stress and Changes in Anxiety- and Depressive-Like Behaviors.eNeuro. 2023 Mar 2;10(3):ENEURO.0300-22.2023. doi: 10.1523/ENEURO.0300-22.2023. Print 2023 Mar. eNeuro. 2023. PMID: 36808099 Free PMC article.
-
Molecular Mechanisms Underlying the Retrieval and Extinction of Morphine Withdrawal-Associated Memories in the Basolateral Amygdala and Dentate Gyrus.Biomedicines. 2022 Mar 2;10(3):588. doi: 10.3390/biomedicines10030588. Biomedicines. 2022. PMID: 35327388 Free PMC article.
-
Repetitive and Inflexible Active Coping and Addiction-like Neuroplasticity in Stressed Mice of a Helplessness-Resistant Inbred Strain.Behav Sci (Basel). 2021 Dec 10;11(12):174. doi: 10.3390/bs11120174. Behav Sci (Basel). 2021. PMID: 34940109 Free PMC article.
References
-
- Mumberg D, Lucibello FC, Schuermann M, Muller R (1991) Alternative splicing of fosB transcripts results in differentially expressed mRNAs encoding functionally antagonistic proteins. Genes Dev 5: 1212–1223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

