Impact of light and temperature on the uptake of algal symbionts by coral juveniles
- PMID: 23185603
- PMCID: PMC3504000
- DOI: 10.1371/journal.pone.0050311
Impact of light and temperature on the uptake of algal symbionts by coral juveniles
Abstract
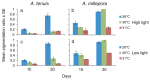
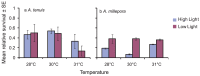
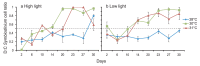
The effects of temperature and light on the breakdown of the coral-Symbiodinium symbiosis are well documented but current understanding of their roles during initial uptake and establishment of symbiosis is limited. In this study, we investigate how temperature and light affect the uptake of the algal symbionts, ITS1 types C1 and D, by juveniles of the broadcast-spawning corals Acropora tenuis and A. millepora. Elevated temperatures had a strong negative effect on Symbiodinium uptake in both coral species, with corals at 31 °C showing as little as 8% uptake compared to 87% at 28 °C. Juveniles in high light treatments (390 µmol photons m(-2) s(-1)) had lower cell counts across all temperatures, emphasizing the importance of the light environment during the initial uptake phase. The proportions of the two Symbiodinium types taken up, as quantified by a real time PCR assay using clade C- and D-specific primers, were also influenced by temperature, although variation in uptake dynamics between the two coral species indicates a host effect. At 28 °C, A. tenuis juveniles were dominated by C1 Symbiodinium, and while the number of D Symbiodinium cells increased at 31 °C, they never exceeded the number of C1 cells. In contrast, juveniles of A. millepora had approximately equal numbers of C1 and D cells at 28 °C, but were dominated by D at 30 °C and 31 °C. This study highlights the significant role that environmental factors play in the establishment of coral-Symbiodinium symbiosis and provides insights into how potentially competing Symbiodinium types take up residence in coral juveniles.
Conflict of interest statement
Figures




References
-
- Muscatine L, Porter JW (1977) Reef corals: mutualistic symbioses adapted to nutrient-poor environments. Bioscience 27: 454–460.
-
- Stanley GD, Fautin DG (2001) The origins of modern corals. Science 291: 1913–1914. - PubMed
-
- Hoegh-Guldberg O (1999) Climate change, coral bleaching and the future of the world's coral reefs. Marine & Freshwater Research 50: 839–866.
-
- Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, et al. (2003) Climate change, human impacts, and the resilience of coral reefs. Science 301: 929–933. - PubMed
-
- Pochon X, Gates RD (2010) A new Symbiodinium clade (Dinophyceae) from soritid foraminifera in Hawai'i. Molecular Phylogenetics and Evolution 56: 492–497. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

