Thermal, chemical and pH induced denaturation of a multimeric β-galactosidase reveals multiple unfolding pathways
- PMID: 23185611
- PMCID: PMC3503960
- DOI: 10.1371/journal.pone.0050380
Thermal, chemical and pH induced denaturation of a multimeric β-galactosidase reveals multiple unfolding pathways
Abstract
Background: In this case study, we analysed the properties of unfolded states and pathways leading to complete denaturation of a multimeric chick pea β-galactosidase (CpGAL), as obtained from treatment with guanidium hydrochloride, urea, elevated temperature and extreme pH.
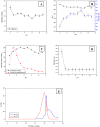
Methodology/principal findings: CpGAL, a heterodimeric protein with native molecular mass of 85 kDa, belongs to α+β class of protein. The conformational stability and thermodynamic parameters of CpGAL unfolding in different states were estimated and interpreted using circular dichroism and fluorescence spectroscopic measurements. The enzyme was found to be structurally and functionally stable in the entire pH range and upto 50 °C temperature. Further increase in temperature induces unfolding followed by aggregation. Chemical induced denaturation was found to be cooperative and transitions were irreversible, non-coincidental and sigmoidal. Free energy of protein unfolding (ΔG(0)) and unfolding constant (K(obs)) were also calculated for chemically denatured CpGAL.
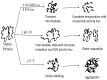
Significance: The protein seems to use different pathways for unfolding in different environments and is a classical example of how the environment dictates the path a protein might take to fold while its amino acid sequence only defines its final three-dimensional conformation. The knowledge accumulated could be of immense biotechnological significance as well.
Conflict of interest statement
Figures



References
-
- Nichtl A, Buchner J, Jaenicke R, Rudolph R, Scheibel T (1998) Folding and association of β-galactosidase. J Mol Biol 282: 1083–1091. - PubMed
-
- Radford SE (2000) Protein folding: progress made and promises ahead. Trends Biochem Sci 25: 611–618. - PubMed
-
- Jagannadham MV, Dubey VK (2003) Differences in the unfolding of procerain induced by pH, guanidine hydrochloride, urea, and temperature. Biochemistry 42: 12287–12297. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

