Peroxiredoxin 1 stimulates endothelial cell expression of VEGF via TLR4 dependent activation of HIF-1α
- PMID: 23185615
- PMCID: PMC3503895
- DOI: 10.1371/journal.pone.0050394
Peroxiredoxin 1 stimulates endothelial cell expression of VEGF via TLR4 dependent activation of HIF-1α
Abstract
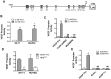
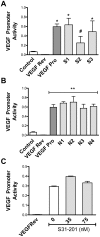
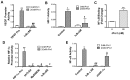
Chronic inflammation leads to the formation of a pro-tumorigenic microenvironment that can promote tumor development, growth and differentiation through augmentation of tumor angiogenesis. Prostate cancer (CaP) risk and prognosis are adversely correlated with a number of inflammatory and angiogenic mediators, including Toll-like receptors (TLRs), NF-κB and vascular endothelial growth factor (VEGF). Peroxiredoxin 1 (Prx1) was recently identified as an endogenous ligand for TLR4 that is secreted from CaP cells and promotes inflammation. Inhibition of Prx1 by CaP cells resulted in reduced expression of VEGF, diminished tumor vasculature and retarded tumor growth. The mechanism by which Prx1 regulates VEGF expression in normoxic conditions was investigated in the current study. Our results show that incubation of mouse vascular endothelial cells with recombinant Prx1 caused increases in VEGF expression that was dependent upon TLR4 and required hypoxia inducible factor-1 (HIF-1) interaction with the VEGF promoter. The induction of VEGF was also dependent upon NF-κB; however, NF-κB interaction with the VEGF promoter was not required for Prx1 induction of VEGF suggesting that NF-κB was acting indirectly to induce VEGF expression. The results presented here show that Prx1 stimulation increased NF-κB interaction with the HIF-1α promoter, leading to enhanced promoter activity and increases in HIF-1α mRNA levels, as well as augmented HIF-1 activity that resulted in VEGF expression. Prx1 induced HIF-1 also promoted NF-κB activity, suggesting the presence of a positive feedback loop that has the potential to perpetuate Prx1 induction of angiogenesis. Strikingly, inhibition of Prx1 expression in CaP was accompanied with reduced expression of HIF-1α. The combined findings of the current study and our previous study suggest that Prx1 interaction with TLR4 promotes CaP growth potentially through chronic activation of tumor angiogenesis.
Conflict of interest statement
Figures

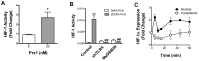





Similar articles
-
A hypoxia-dependent upregulation of hypoxia-inducible factor-1 by nuclear factor-κB promotes gastric tumour growth and angiogenesis.Br J Cancer. 2011 Jan 4;104(1):166-74. doi: 10.1038/sj.bjc.6606020. Epub 2010 Nov 30. Br J Cancer. 2011. PMID: 21119667 Free PMC article.
-
Synergistic up-regulation of vascular endothelial growth factor (VEGF) expression in macrophages by adenosine A2A receptor agonists and endotoxin involves transcriptional regulation via the hypoxia response element in the VEGF promoter.Mol Biol Cell. 2007 Jan;18(1):14-23. doi: 10.1091/mbc.e06-07-0596. Epub 2006 Oct 25. Mol Biol Cell. 2007. PMID: 17065555 Free PMC article.
-
Peroxiredoxin 1 controls prostate cancer growth through Toll-like receptor 4-dependent regulation of tumor vasculature.Cancer Res. 2011 Mar 1;71(5):1637-46. doi: 10.1158/0008-5472.CAN-10-3674. Epub 2011 Feb 22. Cancer Res. 2011. PMID: 21343392 Free PMC article.
-
Chronic and Cycling Hypoxia: Drivers of Cancer Chronic Inflammation through HIF-1 and NF-κB Activation: A Review of the Molecular Mechanisms.Int J Mol Sci. 2021 Oct 2;22(19):10701. doi: 10.3390/ijms221910701. Int J Mol Sci. 2021. PMID: 34639040 Free PMC article. Review.
-
Research progress of HIF-1a on immunotherapy outcomes in immune vascular microenvironment.Front Immunol. 2025 Feb 6;16:1549276. doi: 10.3389/fimmu.2025.1549276. eCollection 2025. Front Immunol. 2025. PMID: 39981236 Free PMC article. Review.
Cited by
-
Shedding light on the molecular and regulatory mechanisms of TLR4 signaling in endothelial cells under physiological and inflamed conditions.Front Immunol. 2023 Nov 24;14:1264889. doi: 10.3389/fimmu.2023.1264889. eCollection 2023. Front Immunol. 2023. PMID: 38077393 Free PMC article. Review.
-
ROS signaling and redox biology in endothelial cells.Cell Mol Life Sci. 2015 Sep;72(17):3281-303. doi: 10.1007/s00018-015-1928-9. Epub 2015 May 14. Cell Mol Life Sci. 2015. PMID: 25972278 Free PMC article. Review.
-
The Autocrine Impact of Nerve Growth Factor on Sheep Uterine Epithelial Cells.Cells. 2025 Jan 31;14(3):208. doi: 10.3390/cells14030208. Cells. 2025. PMID: 39936999 Free PMC article.
-
Identification and verification of PRDX1 as an inflammation marker for colorectal cancer progression.Am J Transl Res. 2016 Feb 15;8(2):842-59. eCollection 2016. Am J Transl Res. 2016. PMID: 27158373 Free PMC article.
-
Essential Roles of Peroxiredoxin IV in Inflammation and Cancer.Molecules. 2022 Oct 2;27(19):6513. doi: 10.3390/molecules27196513. Molecules. 2022. PMID: 36235049 Free PMC article. Review.
References
-
- Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454: 436–444. - PubMed
-
- Shukla S, Maclennan GT, Marengo SR, Resnick MI, Gupta S (2005) Constitutive activation of P I3 K-Akt and NF-kappaB during prostate cancer progression in autochthonous transgenic mouse model. Prostate 64: 224–239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

