Ethylene synthesis and regulated expression of recombinant protein in Synechocystis sp. PCC 6803
- PMID: 23185630
- PMCID: PMC3503970
- DOI: 10.1371/journal.pone.0050470
Ethylene synthesis and regulated expression of recombinant protein in Synechocystis sp. PCC 6803
Abstract
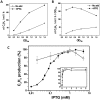
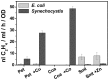
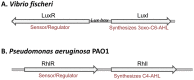
The ethylene-forming enzyme (EFE) from Pseudomonas syringae catalyzes the synthesis of ethylene which can be easily detected in the headspace of closed cultures. A synthetic codon-optimized gene encoding N-terminal His-tagged EFE (EFEh) was expressed in Synechocystis sp. PCC 6803 (Synechocystis) and Escherichia coli (E. coli) under the control of diverse promoters in a self-replicating broad host-range plasmid. Ethylene synthesis was stably maintained in both organisms in contrast to earlier work in Synechococcus elongatus PCC 7942. The rate of ethylene accumulation was used as a reporter for protein expression in order to assess promoter strength and inducibility with the different expression systems. Several metal-inducible cyanobacterial promoters did not function in E. coli but were well-regulated in cyanobacteria, albeit at a low level of expression. The E. coli promoter P(trc) resulted in constitutive expression in cyanobacteria regardless of whether IPTG was added or not. In contrast, a Lac promoter variant, P(A1lacO-1), induced EFE-expression in Synechocystis at a level of expression as high as the Trc promoter and allowed a fine level of IPTG-dependent regulation of protein-expression. The regulation was tight at low cell density and became more relaxed in more dense cultures. A synthetic quorum-sensing promoter system was also constructed and shown to function well in E. coli, however, only a very low level of EFE-activity was observed in Synechocystis, independent of cell density.
Conflict of interest statement
Figures






Similar articles
-
Ethylene production with engineered Synechocystis sp PCC 6803 strains.Microb Cell Fact. 2017 Feb 23;16(1):34. doi: 10.1186/s12934-017-0645-5. Microb Cell Fact. 2017. PMID: 28231787 Free PMC article.
-
A Genetic Toolbox for Modulating the Expression of Heterologous Genes in the Cyanobacterium Synechocystis sp. PCC 6803.ACS Synth Biol. 2018 Jan 19;7(1):276-286. doi: 10.1021/acssynbio.7b00297. Epub 2017 Dec 22. ACS Synth Biol. 2018. PMID: 29232504
-
Enhanced stable production of ethylene in photosynthetic cyanobacterium Synechococcus elongatus PCC 7942.World J Microbiol Biotechnol. 2019 May 8;35(5):77. doi: 10.1007/s11274-019-2652-7. World J Microbiol Biotechnol. 2019. PMID: 31069553 Free PMC article.
-
A guanidine-degrading enzyme controls genomic stability of ethylene-producing cyanobacteria.Nat Commun. 2021 Aug 26;12(1):5150. doi: 10.1038/s41467-021-25369-x. Nat Commun. 2021. PMID: 34446715 Free PMC article.
-
Expression of ethylene-forming enzyme (EFE) of Pseudomonas syringae pv. glycinea in Trichoderma viride.Appl Microbiol Biotechnol. 2008 Sep;80(4):573-8. doi: 10.1007/s00253-008-1562-7. Epub 2008 Jun 25. Appl Microbiol Biotechnol. 2008. PMID: 18575855
Cited by
-
Hydroponics with Microalgae and Cyanobacteria: Emerging Trends and Opportunities in Modern Agriculture.BioTech (Basel). 2024 Jul 22;13(3):27. doi: 10.3390/biotech13030027. BioTech (Basel). 2024. PMID: 39051342 Free PMC article. Review.
-
Optimizing cyanobacterial product synthesis: Meeting the challenges.Bioengineered. 2016 Nov;7(6):490-496. doi: 10.1080/21655979.2016.1207017. Epub 2016 Jul 15. Bioengineered. 2016. PMID: 27420605 Free PMC article.
-
CfrA, a Novel Carbon Flow Regulator, Adapts Carbon Metabolism to Nitrogen Deficiency in Cyanobacteria.Plant Physiol. 2020 Dec;184(4):1792-1810. doi: 10.1104/pp.20.00802. Epub 2020 Sep 8. Plant Physiol. 2020. PMID: 32900980 Free PMC article.
-
Recent examples of α-ketoglutarate-dependent mononuclear non-haem iron enzymes in natural product biosyntheses.Nat Prod Rep. 2018 Aug 15;35(8):792-837. doi: 10.1039/c7np00067g. Nat Prod Rep. 2018. PMID: 29932179 Free PMC article. Review.
-
Ethylene-forming enzyme and bioethylene production.Biotechnol Biofuels. 2014 Mar 3;7(1):33. doi: 10.1186/1754-6834-7-33. Biotechnol Biofuels. 2014. PMID: 24589138 Free PMC article.
References
-
- Sakai M, Ogawa T, Matsuoka M, Fukuda H (1997) Photosynthetic Conversion of Carbon Dioxide to Ethylene by the Recombinant Cyanobacterium, Synechococcus sp. PCC 7942, Which Harbors a Gene for the Ethylene-Forming Enzyme of Pseudomonas syringae . Journal of Fermentation and Bioengineering 84: 434–443.
-
- Takahama K, Matsuoka M, Nagahama K, Ogawa T (2003) Construction and analysis of a recombinant cyanobacterium expressing a chromosomally inserted gene for an ethylene-forming enzyme at the psbAI locus. J Biosci Bioeng 95: 302–305. - PubMed
-
- Ungerer J, Tao L, Davis M, Ghirardi M, Maness P-C, et al. (2012) Sustained photosynthetic conversion of CO2 to ethylene in recombinant cyanobacterium Synechocystis 6803. Energy & Environmental Science.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

