PPARγ agonists promote oligodendrocyte differentiation of neural stem cells by modulating stemness and differentiation genes
- PMID: 23185633
- PMCID: PMC3503969
- DOI: 10.1371/journal.pone.0050500
PPARγ agonists promote oligodendrocyte differentiation of neural stem cells by modulating stemness and differentiation genes
Abstract
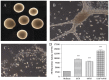
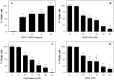
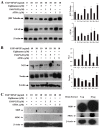
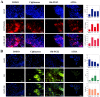
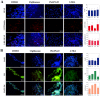
Neural stem cells (NSCs) are a small population of resident cells that can grow, migrate and differentiate into neuro-glial cells in the central nervous system (CNS). Peroxisome proliferator-activated receptor gamma (PPARγ) is a nuclear receptor transcription factor that regulates cell growth and differentiation. In this study we analyzed the influence of PPARγ agonists on neural stem cell growth and differentiation in culture. We found that in vitro culture of mouse NSCs in neurobasal medium with B27 in the presence of epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) induced their growth and expansion as neurospheres. Addition of all-trans retinoic acid (ATRA) and PPARγ agonist ciglitazone or 15-Deoxy-Δ(12,14)-Prostaglandin J(2) (15d-PGJ2) resulted in a dose-dependent inhibition of cell viability and proliferation of NSCs in culture. Interestingly, NSCs cultured with PPARγ agonists, but not ATRA, showed significant increase in oligodendrocyte precursor-specific O4 and NG2 reactivity with a reduction in NSC marker nestin, in 3-7 days. In vitro treatment with PPARγ agonists and ATRA also induced modest increase in the expression of neuronal β-III tubulin and astrocyte-specific GFAP in NSCs in 3-7 days. Further analyses showed that PPARγ agonists and ATRA induced significant alterations in the expression of many stemness and differentiation genes associated with neuro-glial differentiation in NSCs. These findings highlight the influence of PPARγ agonists in promoting neuro-glial differentiation of NSCs and its significance in the treatment of neurodegenerative diseases.
Conflict of interest statement
Figures

References
-
- Miller FD, Gauthier AS (2007) Timing is everything: making neurons versus glia in the developing cortex. Neuron 54: 357–369. - PubMed
-
- Weiss S, Reynolds BA, Vescovi AL, Morshead C, Craig CG, et al. (1996) Is there a neural stem cell in the mammalian forebrain? Trends Neurosci 19: 387–393. - PubMed
-
- Gage FH (2000) Mammalian neural stem cells. Science 287: 1433–1438. - PubMed
-
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, et al. (1998) Neurogenesis in the adult human hippocampus. Nat Med 4: 1313–1317. - PubMed
-
- Gage FH (1998) Stem cells of the central nervous system. Curr Opin Neurobiol 8: 671–676. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

