The gamma chain subunit of Fc receptors is required for alpha-synuclein-induced pro-inflammatory signaling in microglia
- PMID: 23186369
- PMCID: PMC3526448
- DOI: 10.1186/1742-2094-9-259
The gamma chain subunit of Fc receptors is required for alpha-synuclein-induced pro-inflammatory signaling in microglia
Abstract
Background: The protein alpha-synuclein (α-SYN), which is found in the Lewy bodies of dopamine-producing (DA) neurons in the substantia nigra (SN), has an important role in the pathogenesis of Parkinson's disease (PD). Previous studies have shown that neuroinflammation plays a key role in PD pathogenesis. In an AAV-synuclein mouse model of PD, we have found that over-abundance of α-SYN triggers the expression of NF-κB p65, and leads to microglial activation and DA neurodegeneration. We also have observed that Fcγ receptors (FcγR), proteins present on the surface of microglia that bind immunoglobulin G (IgG) and other ligands, are key modulators of α-SYN-induced neurodegeneration.
Methods: In order to study the role of FcγRs in the interactions of α-SYN and microglia, we treated the primary microglial cultures from wild-type (WT) and FcγR-/- mice with aggregated human α-SYN in vitro.
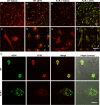
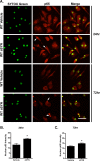
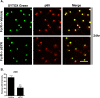
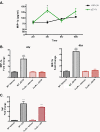
Results: Using immunocytochemistry, we found that α-SYN was taken up by both WT and FcγR-/- microglia, however, their patterns of internalization were different, with aggregation in autophagosomes in WT cells and more diffuse localization in FcγR-/- microglia. In WT microglia, α-SYN induced the nuclear accumulation of NF-κB p65 protein and downstream chemokine expression while in FcγR-/- mouse microglia, α-SYN failed to trigger the enhancement of nuclear NF-κB p65, and the pro-inflammatory signaling was reduced.
Conclusions: Our results suggest that α-SYN can interact directly with microglia and can be internalized and trafficked to autophagosomes. FcγRs mediate this interaction, and in the absence of the gamma chain, there is altered intracellular trafficking and attenuation of pro-inflammatory NF-κB signaling. Therefore, blocking either FcγR signaling or downstream NF-κB activation may be viable therapeutic strategies in PD.
Figures
References
-
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. - DOI - PubMed
-
- Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–192. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

