Tranexamic acid concentrations associated with human seizures inhibit glycine receptors
- PMID: 23187124
- PMCID: PMC3533541
- DOI: 10.1172/JCI63375
Tranexamic acid concentrations associated with human seizures inhibit glycine receptors
Abstract
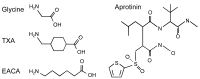
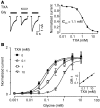
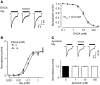
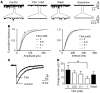
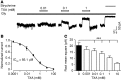
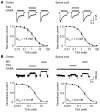
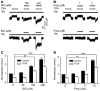
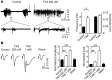
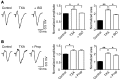
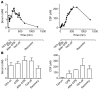
Antifibrinolytic drugs are widely used to reduce blood loss during surgery. One serious adverse effect of these drugs is convulsive seizures; however, the mechanisms underlying such seizures remain poorly understood. The antifibrinolytic drugs tranexamic acid (TXA) and ε-aminocaproic acid (EACA) are structurally similar to the inhibitory neurotransmitter glycine. Since reduced function of glycine receptors causes seizures, we hypothesized that TXA and EACA inhibit the activity of glycine receptors. Here we demonstrate that TXA and EACA are competitive antagonists of glycine receptors in mice. We also showed that the general anesthetic isoflurane, and to a lesser extent propofol, reverses TXA inhibition of glycine receptor-mediated current, suggesting that these drugs could potentially be used to treat TXA-induced seizures. Finally, we measured the concentration of TXA in the cerebrospinal fluid (CSF) of patients undergoing major cardiovascular surgery. Surprisingly, peak TXA concentration in the CSF occurred after termination of drug infusion and in one patient coincided with the onset of seizures. Collectively, these results show that concentrations of TXA equivalent to those measured in the CSF of patients inhibited glycine receptors. Furthermore, isoflurane or propofol may prevent or reverse TXA-induced seizures.
Figures
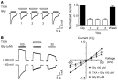
Comment in
-
Understanding the TXA seizure connection.J Clin Invest. 2012 Dec;122(12):4339-41. doi: 10.1172/JCI66724. Epub 2012 Nov 26. J Clin Invest. 2012. PMID: 23187134 Free PMC article.
References
-
- Rahman Z, Hoque R, Ali A, Rahman M, Rahman MS. Blood conservation strategies for reducing peri-operative blood loss in open heart surgery. Mymensingh Med J. 2011;20(1):45–53. - PubMed

