The mitochondrial heme exporter FLVCR1b mediates erythroid differentiation
- PMID: 23187127
- PMCID: PMC3533534
- DOI: 10.1172/JCI62422
The mitochondrial heme exporter FLVCR1b mediates erythroid differentiation
Abstract
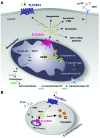
Feline leukemia virus subgroup C receptor 1 (FLVCR1) is a cell membrane heme exporter that maintains the balance between heme levels and globin synthesis in erythroid precursors. It was previously shown that Flvcr1-null mice died in utero due to a failure of erythropoiesis. Here, we identify Flvcr1b, a mitochondrial Flvcr1 isoform that promotes heme efflux into the cytoplasm. Flvcr1b overexpression promoted heme synthesis and in vitro erythroid differentiation, whereas silencing of Flvcr1b caused mitochondrial heme accumulation and termination of erythroid differentiation. Furthermore, mice lacking the plasma membrane isoform (Flvcr1a) but expressing Flvcr1b had normal erythropoiesis, but exhibited hemorrhages, edema, and skeletal abnormalities. Thus, FLVCR1b regulates erythropoiesis by controlling mitochondrial heme efflux, whereas FLVCR1a expression is required to prevent hemorrhages and edema. The aberrant expression of Flvcr1 isoforms may play a role in the pathogenesis of disorders characterized by an imbalance between heme and globin synthesis.
Figures
Comment in
-
Mitochondrial heme: an exit strategy at last.J Clin Invest. 2012 Dec;122(12):4328-30. doi: 10.1172/JCI66607. Epub 2012 Nov 26. J Clin Invest. 2012. PMID: 23187133 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

