Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation
- PMID: 23187627
- PMCID: PMC3514498
- DOI: 10.1038/ncomms2230
Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation
Abstract
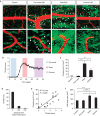
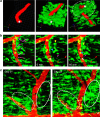
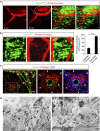
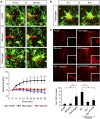
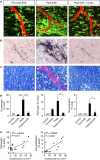
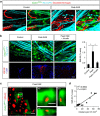
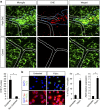
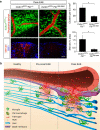
Blood-brain barrier disruption, microglial activation and neurodegeneration are hallmarks of multiple sclerosis. However, the initial triggers that activate innate immune responses and their role in axonal damage remain unknown. Here we show that the blood protein fibrinogen induces rapid microglial responses toward the vasculature and is required for axonal damage in neuroinflammation. Using in vivo two-photon microscopy, we demonstrate that microglia form perivascular clusters before myelin loss or paralysis onset and that, of the plasma proteins, fibrinogen specifically induces rapid and sustained microglial responses in vivo. Fibrinogen leakage correlates with areas of axonal damage and induces reactive oxygen species release in microglia. Blocking fibrin formation with anticoagulant treatment or genetically eliminating the fibrinogen binding motif recognized by the microglial integrin receptor CD11b/CD18 inhibits perivascular microglial clustering and axonal damage. Thus, early and progressive perivascular microglial clustering triggered by fibrinogen leakage upon blood-brain barrier disruption contributes to axonal damage in neuroinflammatory disease.
Conflict of interest statement
H. Lundbeck A/S is sponsoring research in Dr Akassoglou's laboratory to identify therapeutic candidates for neurological diseases. Dr Akassoglou is also a consultant for H. Lundbeck A/S.
Figures
References
-
- Lassmann H., Bruck W., Lucchinetti C. Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy. Trends. Mol. Med. 7, 115–121 (2001). - PubMed
-
- Vos C. M. et al. Blood-brain barrier alterations in both focal and diffuse abnormalities on postmortem MRI in multiple sclerosis. Neurobiol. Dis. 20, 953–960 (2005). - PubMed
-
- Garcia A. D., Doan N. B., Imura T., Bush T. G., Sofroniew M. V. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat. Neurosci. 7, 1233–1241 (2004). - PubMed
-
- van der Valk P., Amor S. Preactive lesions in multiple sclerosis. Curr. Opin. Neurol. 22, 207–213 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL096126/HL/NHLBI NIH HHS/United States
- NS052189/NS/NINDS NIH HHS/United States
- R01 NS066361/NS/NINDS NIH HHS/United States
- K12 HD000850/HD/NICHD NIH HHS/United States
- P41 RR004050/RR/NCRR NIH HHS/United States
- NS066361/NS/NINDS NIH HHS/United States
- CA082103/CA/NCI NIH HHS/United States
- NS051470/NS/NINDS NIH HHS/United States
- C06 RR018928/RR/NCRR NIH HHS/United States
- HL096126/HL/NHLBI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- P30 CA082103/CA/NCI NIH HHS/United States
- RR18928/RR/NCRR NIH HHS/United States
- R01 NS052189/NS/NINDS NIH HHS/United States
- R01 NS051470/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

