In vitro antisense therapeutics for a deep intronic mutation causing Neurofibromatosis type 2
- PMID: 23188051
- PMCID: PMC3722955
- DOI: 10.1038/ejhg.2012.261
In vitro antisense therapeutics for a deep intronic mutation causing Neurofibromatosis type 2
Erratum in
- Eur J Hum Genet. 2014 Jan;22(1):153
Abstract
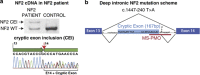
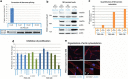
Neurofibromatosis type 2 (NF2) is an autosomal-dominant disorder affecting about 1:33 000 newborns, mainly characterized by the development of tumors of the nervous system and ocular abnormalities. Around 85% of germline NF2 mutations are point mutations. Among them, ∼25% affect splicing and are associated with a variable disease severity. In the context of our NF2 Multidisciplinary Clinics, we have identified a patient fulfilling clinical criteria for the disease and exhibiting a severe phenotype. The patient carries a deep intronic mutation (g. 74409T>A, NG_009057.1) that produces the insertion of a cryptic exon of 167pb in the mature mRNA between exons 13 and 14, resulting in a truncated merlin protein (p.Pro482Profs*39). A mutation-specific antisense phosphorodiamidate morpholino oligomer was designed and used in vitro to effectively restore normal NF2 splicing in patient-derived primary fibroblasts. In addition, merlin protein levels were greatly recovered after morpholino treatment, decreasing patient's fibroblasts in vitro proliferation capacity and restoring cytoeskeleton organization. To our knowledge, this is the first NF2 case caused by a deep intronic mutation in which an in vitro antisense therapeutic approximation has been tested. These results open the possibility of using this approach in vivo for this type of mutation causing NF2.
Figures
Comment in
-
Can manipulation of splicing offer gene therapy possibilities to those with tumour-prone disorders?Eur J Hum Genet. 2013 Jul;21(7):701-2. doi: 10.1038/ejhg.2012.264. Epub 2012 Nov 28. Eur J Hum Genet. 2013. PMID: 23188050 Free PMC article. No abstract available.
Similar articles
-
Antisense therapeutics for neurofibromatosis type 1 caused by deep intronic mutations.Hum Mutat. 2009 Mar;30(3):454-62. doi: 10.1002/humu.20933. Hum Mutat. 2009. PMID: 19241459
-
Antisense oligonucleotides targeting exon 11 are able to partially rescue the NF2-related schwannomatosis phenotype in vitro.Mol Ther Nucleic Acids. 2022 Nov 4;30:493-505. doi: 10.1016/j.omtn.2022.10.026. eCollection 2022 Dec 13. Mol Ther Nucleic Acids. 2022. PMID: 36420221 Free PMC article.
-
[Mutation analysis of NF2 gene and clinical investigation in a Chinese family with neurofibromatosis type II].Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2010 Dec;27(6):688-91. doi: 10.3760/cma.j.issn.1003-9406.2010.06.020. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2010. PMID: 21154335 Chinese.
-
NF2: the wizardry of merlin.Genes Chromosomes Cancer. 2003 Dec;38(4):389-99. doi: 10.1002/gcc.10282. Genes Chromosomes Cancer. 2003. PMID: 14566860 Review.
-
The location of constitutional neurofibromatosis 2 (NF2) splice site mutations is associated with the severity of NF2.J Med Genet. 2005 Jul;42(7):540-6. doi: 10.1136/jmg.2004.029504. J Med Genet. 2005. PMID: 15994874 Free PMC article. Review.
Cited by
-
Can manipulation of splicing offer gene therapy possibilities to those with tumour-prone disorders?Eur J Hum Genet. 2013 Jul;21(7):701-2. doi: 10.1038/ejhg.2012.264. Epub 2012 Nov 28. Eur J Hum Genet. 2013. PMID: 23188050 Free PMC article. No abstract available.
-
Hybridization Capture-Based Next-Generation Sequencing to Evaluate Coding Sequence and Deep Intronic Mutations in the NF1 Gene.Genes (Basel). 2016 Dec 17;7(12):133. doi: 10.3390/genes7120133. Genes (Basel). 2016. PMID: 27999334 Free PMC article.
-
The evolving landscape of NF gene therapy: Hurdles and opportunities.Mol Ther Nucleic Acids. 2025 Feb 3;36(1):102475. doi: 10.1016/j.omtn.2025.102475. eCollection 2025 Mar 11. Mol Ther Nucleic Acids. 2025. PMID: 40034205 Free PMC article. Review.
-
Deep intronic mutations and human disease.Hum Genet. 2017 Sep;136(9):1093-1111. doi: 10.1007/s00439-017-1809-4. Epub 2017 May 12. Hum Genet. 2017. PMID: 28497172 Review.
-
Transposon clusters as substrates for aberrant splice-site activation.RNA Biol. 2021 Mar;18(3):354-367. doi: 10.1080/15476286.2020.1805909. Epub 2020 Sep 23. RNA Biol. 2021. PMID: 32965162 Free PMC article.
References
-
- McClatchey AI, Giovannini M. Membrane organization and tumorigenesis—the NF2 tumor suppressor, Merlin. Genes Dev. 2005;19:2265–2277. - PubMed
-
- Trofatter JA, MacCollin MM, Rutter JL, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;72:791–800. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

