Safety and tolerability of low-dose naltrexone therapy in children with moderate to severe Crohn's disease: a pilot study
- PMID: 23188075
- PMCID: PMC3586944
- DOI: 10.1097/MCG.0b013e3182702f2b
Safety and tolerability of low-dose naltrexone therapy in children with moderate to severe Crohn's disease: a pilot study
Abstract
Background: There is an unmet need for safe and effective medicines to treat children with Crohn's disease. Recently, investigations have shown an association between endogenous opioid peptides and inflammatory cells.
Aims: The aims of this study were to evaluate the safety and tolerability of an opioid antagonist, naltrexone, in children with moderate to severe Crohn's disease.
Methods: A pilot clinical trial was conducted in children with moderate to severe Crohn's disease. Fourteen subjects with a mean age of 12.3 years (range, 8 to 17 y) were enrolled. Children were randomized to placebo or naltrexone (0.1 mg/kg) orally for 8 weeks followed by open-labeled treatment with 8 additional weeks of naltrexone. Safety and toxicity were monitored by physical examinations and blood chemistries. Clinical activity was assessed by the Pediatric Crohn's Disease Activity Index (PCDAI) and Quality of life was monitored by the Impact III survey.
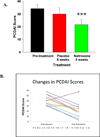
Results: Oral naltrexone was well tolerated without any serious adverse events in children with moderate to severe Crohn's disease. PCDAI scores significantly decreased from pretreatment values (34.2±3.3) with an 8-week course of naltrexone therapy (21.7±3.9) (P=0.005). Twenty-five percent of those treated with naltrexone were considered in remission (score ≤10) and 67% had improved with mild disease activity (decrease in PCDAI score by at least 10 points) at the end of the study. Systemic and social quality of life improved with naltrexone treatment (P=0.035).
Conclusions: Naltrexone therapy seems safe with limited toxicity when given to children with Crohn's disease and may reduce disease activity.
Trial registration: ClinicalTrials.gov NCT00715117.
Conflict of interest statement
Conflict of interest: Dr. Jill Smith has intellectual property rights and is a co-inventor on a US patent for the use of naltrexone in inflammatory bowel disease. This disclosure was provided to all study participants. She has no financial relationships relevant to this article to disclose. The statistical analysis of the data sets relating to efficacy and safety were independently determined by a biostatistician who has no conflict of interest.
Figures


Comment in
-
Avoiding narcotics in Crohn's disease.J Clin Gastroenterol. 2013 Apr;47(4):293-5. doi: 10.1097/MCG.0b013e318278aeec. J Clin Gastroenterol. 2013. PMID: 23426445 No abstract available.
-
Naltrexone therapy for Crohn's disease and ulcerative colitis.J Clin Gastroenterol. 2014 Sep;48(8):742. doi: 10.1097/MCG.0000000000000093. J Clin Gastroenterol. 2014. PMID: 24583747 No abstract available.
References
-
- Hyams J, Crandall W, Kugathasan S, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn's disease in children. Gastroenterology. 2007;132:863–873. - PubMed
-
- Bell S, Kamm MA. Antibodies to tumour necrosis factor alpha as treatment for Crohn's disease. Lancet. 2000;355:858–860. - PubMed
-
- Sandborn WJ, Hanauer SB. Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results, and safety. Inflamm Bowel Dis. 1999;5:119–133. - PubMed
-
- Toruner M, Loftus EV, Jr, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929–936. - PubMed
-
- Mackey AC, Green L, Liang LC, et al. Hepatosplenic T cell lymphoma associated with infliximab use in young patients treated for inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;44:265–267. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

