Skin squamous cell carcinoma propagating cells increase with tumour progression and invasiveness
- PMID: 23188079
- PMCID: PMC3545290
- DOI: 10.1038/emboj.2012.312
Skin squamous cell carcinoma propagating cells increase with tumour progression and invasiveness
Abstract
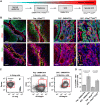
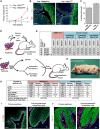
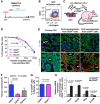
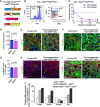
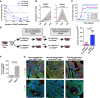
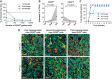
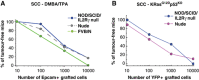
Cancer stem cells have been described in various cancers including squamous tumours of the skin by their ability to reform secondary tumours upon transplantation into immunodeficient mice. Here, we used transplantation of limiting dilution of different populations of FACS-isolated tumour cells from four distinct mouse models of squamous skin tumours to investigate the frequency of tumour propagating cells (TPCs) at different stages of tumour progression. We found that benign papillomas, despite growing rapidly in vivo and being clonogenic in vitro, reformed secondary tumours upon transplantation at very low frequency and only when tumour cells were co-transplanted together with tumour-associated fibroblasts or endothelial cells. In two models of skin squamous cell carcinoma (SCC), TPCs increased with tumour invasiveness. Interestingly, the frequency of TPCs increased in CD34(HI) but not in CD34(LO) SCC cells with serial transplantations, while the two populations initially gave rise to secondary tumours with the same frequency. Our results illustrate the progressive increase of squamous skin TPCs with tumour progression and invasiveness and reveal that serial transplantation may be required to define the long-term renewal potential of TPCs.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Alam M, Ratner D (2001) Cutaneous squamous-cell carcinoma. N Engl J Med 344: 975–983 - PubMed
-
- Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, Fauvet F, Puisieux I, Doglioni C, Piccinin S, Maestro R, Voeltzel T, Selmi A, Valsesia-Wittmann S, Caron de Fromentel C, Puisieux A (2008) Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell 14: 79–89 - PubMed
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007 - PubMed
-
- Barrett LE, Granot Z, Coker C, Iavarone A, Hambardzumyan D, Holland EC, Nam HS, Benezra R (2012) Self-renewal does not predict tumor growth potential in mouse models of high-grade glioma. Cancer Cell 21: 11–24 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

