The alarmin Mrp8/14 as regulator of the adaptive immune response during allergic contact dermatitis
- PMID: 23188082
- PMCID: PMC3545303
- DOI: 10.1038/emboj.2012.309
The alarmin Mrp8/14 as regulator of the adaptive immune response during allergic contact dermatitis
Abstract
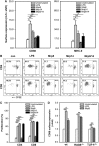
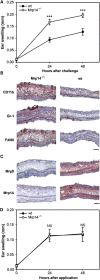
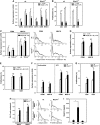
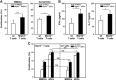
Mrp8 and Mrp14 are endogenous alarmins amplifying inflammation via Toll-like receptor-4 (TLR-4) activation. Due to their pro-inflammatory properties, alarmins are supposed to enhance adaptive immunity via activation of dendritic cells (DCs). In contrast, analysing a model of allergic contact dermatitis (ACD) we observed a more severe disease outcome in Mrp8/14-deficient compared to wild-type mice. This unexpected phenotype was associated with an enhanced T-cell response due to an accelerated maturation of DCs in Mrp8/14-deficient mice. Accordingly, Mrp8, the active component of the heterocomplex, inhibits early DC maturation and antigen presentation in a TLR-4-dependent manner. Transfer of DCs purified from the local lymph nodes of sensitized Mrp8/14-deficient to wild-type mice determined the outcome of ACD. Our results link a pro-inflammatory role of the endogenous TLR-4 ligand Mrp8/14 to a regulatory function in adaptive immunity, which shows some similarities with the 'hygiene hypothesis' regarding continuous TLR-4 stimulation and decreased risk of allergy.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Injury-induced MRP8/MRP14 stimulates IP-10/CXCL10 in monocytes/macrophages.FASEB J. 2015 Jan;29(1):250-62. doi: 10.1096/fj.14-255992. Epub 2014 Oct 23. FASEB J. 2015. PMID: 25342131 Free PMC article.
-
Alarmins MRP8 and MRP14 induce stress tolerance in phagocytes under sterile inflammatory conditions.Cell Rep. 2014 Dec 24;9(6):2112-23. doi: 10.1016/j.celrep.2014.11.020. Epub 2014 Dec 11. Cell Rep. 2014. PMID: 25497086
-
Deficiency of myeloid-related proteins 8 and 14 (Mrp8/Mrp14) does not block inflammaging but prevents steatosis.Oncotarget. 2016 Jun 14;7(24):35535-35551. doi: 10.18632/oncotarget.9550. Oncotarget. 2016. PMID: 27224926 Free PMC article.
-
Expression of the calcium-binding proteins MRP8 and MRP14 by early infiltrating cells in experimental contact dermatitis.Int Arch Allergy Immunol. 1992;98(2):140-5. doi: 10.1159/000236177. Int Arch Allergy Immunol. 1992. PMID: 1643439
-
The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells.Nat Med. 2010 Jun;16(6):713-7. doi: 10.1038/nm.2150. Epub 2010 May 9. Nat Med. 2010. PMID: 20473308
Cited by
-
Serum Calprotectin, a Marker of Neutrophil Activation, and Other Mediators of Inflammation in Response to Various Types of Extreme Physical Exertion in Healthy Volunteers.J Inflamm Res. 2020 May 22;13:223-231. doi: 10.2147/JIR.S250675. eCollection 2020. J Inflamm Res. 2020. PMID: 32547154 Free PMC article.
-
MRP8/14 Is a Molecular Signature Triggered by Dopamine in HIV Latent Myeloid Targets That Increases HIV Transcription and Distinguishes HIV+ Methamphetamine Users with Detectable CSF Viral Load and Brain Pathology.Viruses. 2023 Jun 13;15(6):1363. doi: 10.3390/v15061363. Viruses. 2023. PMID: 37376663 Free PMC article.
-
Role of Calprotectin as a Biomarker in Periodontal Disease.Mediators Inflamm. 2019 Aug 21;2019:3515026. doi: 10.1155/2019/3515026. eCollection 2019. Mediators Inflamm. 2019. PMID: 31530995 Free PMC article. Review.
-
S100-Alarmins Are Essential Pilots of Postnatal Innate Immune Adaptation.Front Immunol. 2020 Apr 30;11:688. doi: 10.3389/fimmu.2020.00688. eCollection 2020. Front Immunol. 2020. PMID: 32425933 Free PMC article. Review.
-
Serum Calprotectin Levels in Vitiligo Patients and Disease Relation.Dermatol Pract Concept. 2024 Jul 1;14(3):e2024184. doi: 10.5826/dpc.1403a184. Dermatol Pract Concept. 2024. PMID: 39122544 Free PMC article.
References
-
- Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392: 245–252 - PubMed
-
- Bour H, Peyron E, Gaucherand M, Garrigue J, Desvignes C, Kaiserlian D, Revillard J, Nicolas J (2005) Major histocompatibility complex class I-restricted CD8+ T cells and class II-restricted CD4+ T cells, respectively, mediate and regulate contact sensitivity to dinitrofluorobenzene. Eur J Immunol 25: 3006–3010 - PubMed
-
- Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, Roth J, Gabrilovich DI (2008) Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med 205: 2235–2249 - PMC - PubMed
-
- Ege MJ, Mayer M, Normand A, Genuneit J, Cookson W, Braun-Fahrländer C, Heederik D, Piarroux R, von Mutius E (2011) Exposure to environmental microorganisms and childhood asthma. N Engl J Med 364: 701–709 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

