Binding determinants of the small heat shock protein, αB-crystallin: recognition of the 'IxI' motif
- PMID: 23188086
- PMCID: PMC3545294
- DOI: 10.1038/emboj.2012.318
Binding determinants of the small heat shock protein, αB-crystallin: recognition of the 'IxI' motif
Abstract
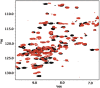
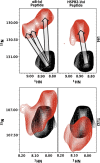
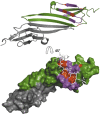
Small heat shock proteins (sHSPs) play a central role in protein homeostasis under conditions of stress by binding partly unfolded, aggregate-prone proteins and keeping them soluble. Like many sHSPs, the widely expressed human sHSP, αB-crystallin ('αB'), forms large polydisperse multimeric assemblies. Molecular interactions involved in both sHSP function and oligomer formation remain to be delineated. A growing database of structural information reveals that a central conserved α-crystallin domain (ACD) forms dimeric building blocks, while flanking N- and C-termini direct the formation of larger sHSP oligomers. The most commonly observed inter-subunit interaction involves a highly conserved C-terminal 'IxI/V' motif and a groove in the ACD that is also implicated in client binding. To investigate the inherent properties of this interaction, peptides mimicking the IxI/V motif of αB and other human sHSPs were tested for binding to dimeric αB-ACD. IxI-mimicking peptides bind the isolated ACD at 22°C in a manner similar to interactions observed in the oligomer at low temperature, confirming these interactions are likely to exist in functional αB oligomers.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
The IXI/V motif in the C-terminal extension of alpha-crystallins: alternative interactions and oligomeric assemblies.Mol Vis. 2004 Sep 8;10:655-62. Mol Vis. 2004. PMID: 15448619
-
N-terminal domain of alphaB-crystallin provides a conformational switch for multimerization and structural heterogeneity.Proc Natl Acad Sci U S A. 2011 Apr 19;108(16):6409-14. doi: 10.1073/pnas.1014656108. Epub 2011 Apr 4. Proc Natl Acad Sci U S A. 2011. PMID: 21464278 Free PMC article.
-
The functional roles of the unstructured N- and C-terminal regions in αB-crystallin and other mammalian small heat-shock proteins.Cell Stress Chaperones. 2017 Jul;22(4):627-638. doi: 10.1007/s12192-017-0789-6. Epub 2017 Apr 8. Cell Stress Chaperones. 2017. PMID: 28391594 Free PMC article.
-
One size does not fit all: the oligomeric states of αB crystallin.FEBS Lett. 2013 Apr 17;587(8):1073-80. doi: 10.1016/j.febslet.2013.01.021. Epub 2013 Jan 20. FEBS Lett. 2013. PMID: 23340341 Free PMC article. Review.
-
The multifaceted nature of αB-crystallin.Cell Stress Chaperones. 2020 Jul;25(4):639-654. doi: 10.1007/s12192-020-01098-w. Epub 2020 May 7. Cell Stress Chaperones. 2020. PMID: 32383140 Free PMC article. Review.
Cited by
-
A weakened interface in the P182L variant of HSP27 associated with severe Charcot-Marie-Tooth neuropathy causes aberrant binding to interacting proteins.EMBO J. 2021 Apr 15;40(8):e103811. doi: 10.15252/embj.2019103811. Epub 2021 Mar 1. EMBO J. 2021. PMID: 33644875 Free PMC article.
-
α-Crystallin Domains of Five Human Small Heat Shock Proteins (sHsps) Differ in Dimer Stabilities and Ability to Incorporate Themselves into Oligomers of Full-Length sHsps.Int J Mol Sci. 2023 Jan 6;24(2):1085. doi: 10.3390/ijms24021085. Int J Mol Sci. 2023. PMID: 36674601 Free PMC article.
-
Chaperone-like activity of the N-terminal region of a human small heat shock protein and chaperone-functionalized nanoparticles.Proteins. 2019 May;87(5):401-415. doi: 10.1002/prot.25662. Epub 2019 Feb 7. Proteins. 2019. PMID: 30684363 Free PMC article.
-
A Study on the Temperature-Dependent Behavior of Small Heat Shock Proteins from Methanogens.Int J Mol Sci. 2025 Jun 16;26(12):5748. doi: 10.3390/ijms26125748. Int J Mol Sci. 2025. PMID: 40565211 Free PMC article.
-
Evolution of crystallins for a role in the vertebrate eye lens.Protein Sci. 2013 Apr;22(4):367-80. doi: 10.1002/pro.2229. Epub 2013 Feb 26. Protein Sci. 2013. PMID: 23389822 Free PMC article. Review.
References
-
- Bagnéris C, Bateman OA, Naylor CE, Cronin N, Boelens WC, Keep NH, Slingsby C (2009) Crystal structures of alpha-crystallin domain dimers of alphaB-crystallin and Hsp20. J Mol Biol 392: 1242–1252 - PubMed
-
- Baldwin AJ, Hilton GR, Lioe H, Bagneris C, Benesch JLP, Kay LE (2011) Quaternary dynamics of αB-crystallin as a direct consequence of localised tertiary fluctuations in the C-terminus. J Mol Biol 413: 310–320 - PubMed
-
- Baldwin AJ, Lioe H, Hilton GR, Baker LA, Rubinstein JL, Kay LE, Benesch J (2011) The polydispersity of aB-crystallin is rationalized by an interconverting polyhedral architecture. Structure 19: 1855–1863 - PubMed
-
- Baranova EV, Weeks SD, Beelen S, Bukach OV, Gusev NB, Strelkov SV (2011) Three-dimensional structure of α-crystallin domain dimers of human small heat shock proteins HSPB1 and HSPB6. J Mol Biol 411: 110–122 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

