Simvastatin ameliorates cauda equina compression injury in a rat model of lumbar spinal stenosis
- PMID: 23188522
- PMCID: PMC3587651
- DOI: 10.1007/s11481-012-9419-3
Simvastatin ameliorates cauda equina compression injury in a rat model of lumbar spinal stenosis
Abstract
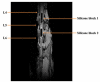
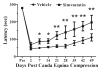
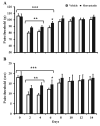
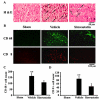
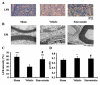
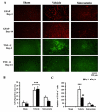
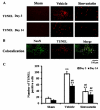
Lumbar spinal stenosis (LSS) is the leading cause of morbidity and mortality worldwide. LSS pathology is associated with secondary injury caused by inflammation, oxidative damage and cell death. Apart from laminectomy, pharmacological therapy targeting secondary injury is limited. Statins are FDA-approved cholesterol-lowering drug. They also show pleiotropic anti-inflammatory, antioxidant and neuroprotective effects. To investigate the therapeutic efficacy of simvastatin in restoring normal locomotor function after cauda equina compression (CEC) in a rat model of LSS, CEC injury was induced in rats by implanting silicone gels into the epidural spaces of L4 and L6. Experimental group was treated with simvastatin (5 mg/kg body weight), while the injured (vehicle) and sham operated (sham) groups received vehicle solution. Locomotor function in terms of latency on rotarod was measured for 49 days and the threshold of pain was determined for 14 days. Rats were sacrificed on day 3 and 14 and the spinal cord and cauda equina fibers were extracted and studied by histology, immunofluorescence, electron microscopy (EM) and TUNEL assay. Simvastatin aided locomotor functional recovery and enhanced the threshold of pain after the CEC. Cellular Infiltration and demyelination decreased in the spinal cord from the simvastatin group. EM revealed enhanced myelination of cauda equina in the simvastatin group. TUNEL assay showed significantly decreased number of apoptotic neurons in spinal cord from the simvastatin group compared to the vehicle group. Simvastatin hastens the locomotor functional recovery and reduces pain after CEC. These outcomes are mediated through the neuroprotective and anti-inflammatory properties of simvastatin. The data indicate that simvastatin may be a promising drug candidate for LSS treatment in humans.
Figures
Similar articles
-
Neuroprotective effect of hydrogen sulfide on acute cauda equina injury in rats.Spine J. 2016 Mar;16(3):402-7. doi: 10.1016/j.spinee.2015.10.046. Epub 2015 Oct 30. Spine J. 2016. PMID: 26523961
-
S-Nitrosoglutathione administration ameliorates cauda equina compression injury in rats.Neurosci Med. 2012 Sep 25;3(3):294-305. doi: 10.4236/nm.2012.33034. Neurosci Med. 2012. PMID: 23997981 Free PMC article.
-
Oral administration of cytosolic PLA2 inhibitor arachidonyl trifluoromethyl ketone ameliorates cauda equina compression injury in rats.J Neuroinflammation. 2015 May 15;12:94. doi: 10.1186/s12974-015-0311-y. J Neuroinflammation. 2015. PMID: 25971887 Free PMC article.
-
Expression of Nogo-A in dorsal root ganglion in rats with cauda equina injury.Biochem Biophys Res Commun. 2020 Jun 18;527(1):131-137. doi: 10.1016/j.bbrc.2020.04.094. Epub 2020 Apr 28. Biochem Biophys Res Commun. 2020. PMID: 32446356
-
Lumbosacral stenosis and injury of the cauda equina.Vet Clin North Am Small Anim Pract. 1988 May;18(3):697-710. doi: 10.1016/s0195-5616(88)50062-1. Vet Clin North Am Small Anim Pract. 1988. PMID: 3289251 Review.
Cited by
-
Animal Models of Intervertebral Disc Diseases: Advantages, Limitations, and Future Directions.Neurol Int. 2024 Dec 9;16(6):1788-1818. doi: 10.3390/neurolint16060129. Neurol Int. 2024. PMID: 39728755 Free PMC article. Review.
-
Quantification of edematous changes by diffusion magnetic resonance imaging in gastrocnemius muscles after spinal nerve ligation.PLoS One. 2018 Feb 22;13(2):e0193306. doi: 10.1371/journal.pone.0193306. eCollection 2018. PLoS One. 2018. PMID: 29470522 Free PMC article.
-
Lysophosphatidic Acid Induced Apoptosis, DNA Damage, and Oxidative Stress in Spinal Cord Neurons by Upregulating LPA4/LPA6 Receptors.Mediators Inflamm. 2022 Sep 30;2022:1818758. doi: 10.1155/2022/1818758. eCollection 2022. Mediators Inflamm. 2022. PMID: 36248188 Free PMC article.
-
Amelioration of spinal cord injury in rats by blocking peroxynitrite/calpain activity.BMC Neurosci. 2018 Aug 13;19(1):50. doi: 10.1186/s12868-018-0450-z. BMC Neurosci. 2018. PMID: 30103682 Free PMC article.
-
Wheelchair use and lipophilic statin medications may influence bone loss in chronic spinal cord injury: findings from the FRASCI-bone loss study.Osteoporos Int. 2016 Dec;27(12):3503-3511. doi: 10.1007/s00198-016-3678-4. Epub 2016 Jul 13. Osteoporos Int. 2016. PMID: 27412619 Free PMC article.
References
-
- Abrahamson EE, Ikonomovic MD, Dixon CE, DeKosky ST. Simvastatin therapy prevents brain trauma-induced increases in beta-amyloid peptide levels. Ann Neurol. 2009;66(3):407–414. doi:10.1002/ana.21731. - PubMed
-
- Bethea JR. Spinal cord injury-induced inflammation: a dualedged sword. Prog Brain Res. 2000;128:33–42. doi:10.1016/S0079-6123(00)28005-9. - PubMed
-
- Bresnahan L, Fessler RG, Natarajan RN. Evaluation of change in muscle activity as a result of posterior lumbar spine surgery using a dynamic modeling system. Spine (Phila Pa 1976) 2010;35(16):E761–E767. doi:10.1097/BRS.0b013e3181e45a6e. - PubMed
-
- Brosamle C, Schwab ME. Ipsilateral, ventral corticospinal tract of the adult rat: ultrastructure, myelination and synaptic connections. J Neurocytol. 2000;29(7):499–507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 DC000422/DC/NIDCD NIH HHS/United States
- R01 NS022576/NS/NINDS NIH HHS/United States
- R37 NS022576/NS/NINDS NIH HHS/United States
- NS-72511/NS/NINDS NIH HHS/United States
- C06 RR018823/RR/NCRR NIH HHS/United States
- C06 RR015455/RR/NCRR NIH HHS/United States
- R01 NS072511/NS/NINDS NIH HHS/United States
- NS-37766/NS/NINDS NIH HHS/United States
- CO6 RR0015455/CO/NCI NIH HHS/United States
- DC00422/DC/NIDCD NIH HHS/United States
- NS-22576/NS/NINDS NIH HHS/United States
- CO6 RR018823/CO/NCI NIH HHS/United States
- R01 NS037766/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

