Small misfolded Tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons
- PMID: 23188818
- PMCID: PMC3548495
- DOI: 10.1074/jbc.M112.394528
Small misfolded Tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons
Abstract
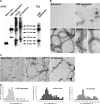
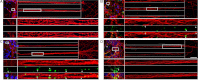
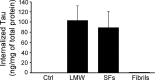
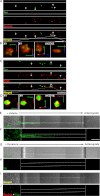
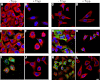
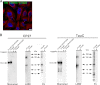
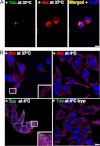
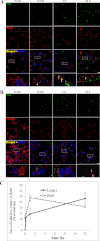
The accumulation of Tau into aggregates is associated with key pathological events in frontotemporal lobe degeneration (FTD-Tau) and Alzheimer disease (AD). Recent data have shown that misfolded Tau can be internalized by cells in vitro (Frost, B., Jacks, R. L., and Diamond, M. I. (2009) J. Biol. Chem. 284, 12845-12852) and propagate pathology in vivo (Clavaguera, F., Bolmont, T., Crowther, R. A., Abramowski, D., Frank, S., Probst, A., Fraser, G., Stalder, A. K., Beibel, M., Staufenbiel, M., Jucker, M., Goedert, M., and Tolnay, M. (2009) Nat. Cell Biol. 11, 909-913; Lasagna-Reeves, C. A., Castillo-Carranza, D. L., Sengupta, U., Guerrero-Munoz, M. J., Kiritoshi, T., Neugebauer, V., Jackson, G. R., and Kayed, R. (2012) Sci. Rep. 2, 700). Here we show that recombinant Tau misfolds into low molecular weight (LMW) aggregates prior to assembly into fibrils, and both extracellular LMW Tau aggregates and short fibrils, but not monomers, long fibrils, nor long filaments purified from brain extract are taken up by neurons. Remarkably, misfolded Tau can be internalized at the somatodendritic compartment, or the axon terminals and it can be transported anterogradely, retrogradely, and can enhance tauopathy in vivo. The internalized Tau aggregates co-localize with dextran, a bulk-endocytosis marker, and with the endolysosomal compartments. Our findings demonstrate that exogenous Tau can be taken up by cells, uptake depends on both the conformation and size of the Tau aggregates and once inside cells, Tau can be transported. These data provide support for observations that tauopathy can spread trans-synaptically in vivo, via cell-to-cell transfer.
Figures




References
-
- Ballatore C., Lee V. M., Trojanowski J. Q. (2007) Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat. Rev. Neurosci. 8, 663–672 - PubMed
-
- Hutton M., Lendon C. L., Rizzu P., Baker M., Froelich S., Houlden H., Pickering-Brown S., Chakraverty S., Isaacs A., Grover A., Hackett J., Adamson J., Lincoln S., Dickson D., Davies P., Petersen R. C., Stevens M., de Graaff E., Wauters E., van Baren J., Hillebrand M., Joosse M., Kwon J. M., Nowotny P., Che L. K., Norton J., Morris J. C., Reed L. A., Trojanowski J., Basun H., Lannfelt L., Neystat M., Fahn S., Dark F., Tannenberg T., Dodd P. R., Hayward N., Kwok J. B., Schofield P. R., Andreadis A., Snowden J., Craufurd D., Neary D., Owen F., Oostra B. A., Hardy J., Goate A., van Swieten J., Mann D., Lynch T., Heutink P. (1998) Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393, 702–705 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

