Direct regulation of nephrin tyrosine phosphorylation by Nck adaptor proteins
- PMID: 23188823
- PMCID: PMC3548463
- DOI: 10.1074/jbc.M112.439463
Direct regulation of nephrin tyrosine phosphorylation by Nck adaptor proteins
Abstract
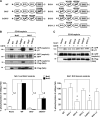
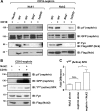
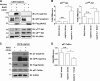
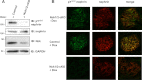
The transmembrane protein nephrin is a key component of the kidney slit diaphragm that contributes to the morphology of podocyte foot processes through signaling to the underlying actin cytoskeleton. We have recently reported that tyrosine phosphorylation of the cytoplasmic tail of nephrin facilitates recruitment of Nck SH2/SH3 adaptor proteins and subsequent actin remodeling and that phosphorylation of the Nck binding sites on nephrin is decreased during podocyte injury. We now demonstrate that Nck directly modulates nephrin phosphorylation through formation of a signaling complex with the Src family kinase Fyn. The ability of Nck to enhance nephrin phosphorylation is compromised in the presence of a Src family kinase inhibitor and when the SH3 domains of Nck are mutated. Furthermore, induced loss of Nck expression in podocytes in vivo is associated with a rapid reduction in nephrin tyrosine phosphorylation. Our results suggest that Nck may facilitate dynamic signaling events at the slit diaphragm by promoting Fyn-dependent phosphorylation of nephrin, which may be important in the regulation of foot process morphology and response to podocyte injury.
Figures


Similar articles
-
Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes.Nature. 2006 Apr 6;440(7085):818-23. doi: 10.1038/nature04662. Epub 2006 Mar 8. Nature. 2006. PMID: 16525419
-
Phosphorylation of Nephrin Triggers Ca2+ Signaling by Recruitment and Activation of Phospholipase C-{gamma}1.J Biol Chem. 2009 Mar 27;284(13):8951-62. doi: 10.1074/jbc.M806851200. Epub 2009 Jan 29. J Biol Chem. 2009. PMID: 19179337 Free PMC article.
-
Vascular endothelial growth factor receptor 2 direct interaction with nephrin links VEGF-A signals to actin in kidney podocytes.J Biol Chem. 2011 Nov 18;286(46):39933-44. doi: 10.1074/jbc.M111.241620. Epub 2011 Sep 21. J Biol Chem. 2011. PMID: 21937443 Free PMC article.
-
The SH2 and SH3 adapter Nck: a two-gene family and a linker between tyrosine kinases and multiple signaling networks.Histol Histopathol. 2000 Jul;15(3):947-55. doi: 10.14670/HH-15.947. Histol Histopathol. 2000. PMID: 10963137 Review.
-
Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton.Trends Cell Biol. 2007 Sep;17(9):428-37. doi: 10.1016/j.tcb.2007.06.006. Epub 2007 Sep 4. Trends Cell Biol. 2007. PMID: 17804239 Review.
Cited by
-
Selective role of Nck1 in atherogenic inflammation and plaque formation.J Clin Invest. 2020 Aug 3;130(8):4331-4347. doi: 10.1172/JCI135552. J Clin Invest. 2020. PMID: 32427580 Free PMC article.
-
Immunopathogenesis of idiopathic nephrotic syndrome with relapse.Semin Immunopathol. 2014 Jul;36(4):421-9. doi: 10.1007/s00281-013-0415-3. Epub 2014 Jan 9. Semin Immunopathol. 2014. PMID: 24402710 Free PMC article. Review.
-
Role of nephrin phosphorylation inducted by dexamethasone and angiotensin II in podocytes.Mol Biol Rep. 2014 Jun;41(6):3591-5. doi: 10.1007/s11033-014-3222-6. Epub 2014 Feb 11. Mol Biol Rep. 2014. PMID: 24515388
-
Nephrin Contributes to Insulin Secretion and Affects Mammalian Target of Rapamycin Signaling Independently of Insulin Receptor.J Am Soc Nephrol. 2016 Apr;27(4):1029-41. doi: 10.1681/ASN.2015020210. Epub 2015 Sep 23. J Am Soc Nephrol. 2016. PMID: 26400569 Free PMC article.
-
Pathogenesis of proteinuria in idiopathic minimal change disease: molecular mechanisms.Pediatr Nephrol. 2016 Dec;31(12):2179-2189. doi: 10.1007/s00467-016-3379-4. Epub 2016 Jul 6. Pediatr Nephrol. 2016. PMID: 27384691 Review.
References
-
- Pavenstädt H., Kriz W., Kretzler M. (2003) Cell biology of the glomerular podocyte. Physiol. Rev. 83, 253–307 - PubMed
-
- Kriz W., Kretzler M., Provoost A. P., Shirato I. (1996) Stability and leakiness: opposing challenges to the glomerulus. Kidney Int. 49, 1570–1574 - PubMed
-
- Welsh G. I., Saleem M. A. (2010) Nephrin—signature molecule of the glomerular podocyte? J. Pathol. 220, 328–337 - PubMed
-
- Verma R., Wharram B., Kovari I., Kunkel R., Nihalani D., Wary K. K., Wiggins R. C., Killen P., Holzman L. B. (2003) Fyn binds to and phosphorylates the kidney slit diaphragm component nephrin. J. Biol. Chem. 278, 20716–20723 - PubMed
-
- Yu C. C., Yen T. S., Lowell C. A., DeFranco A. L. (2001) Lupus-like kidney disease in mice deficient in the Src family tyrosine kinases Lyn and Fyn. Curr. Biol. 11, 34–38 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

