Olivary subthreshold oscillations and burst activity revisited
- PMID: 23189043
- PMCID: PMC3504313
- DOI: 10.3389/fncir.2012.00091
Olivary subthreshold oscillations and burst activity revisited
Abstract
The inferior olive (IO) forms one of the major gateways for information that travels to the cerebellar cortex. Olivary neurons process sensory and motor signals that are subsequently relayed to Purkinje cells. The intrinsic subthreshold membrane potential oscillations of the olivary neurons are thought to be important for gating this flow of information. In vitro studies have revealed that the phase of the subthreshold oscillation determines the size of the olivary burst and may gate the information flow or encode the temporal state of the olivary network. Here, we investigated whether the same phenomenon occurred in murine olivary cells in an intact olivocerebellar system using the in vivo whole-cell recording technique. Our in vivo findings revealed that the number of wavelets within the olivary burst did not encode the timing of the spike relative to the phase of the oscillation but was related to the amplitude of the oscillation. Manipulating the oscillation amplitude by applying Harmaline confirmed the inverse relationship between the amplitude of oscillation and the number of wavelets within the olivary burst. Furthermore, we demonstrated that electrotonic coupling between olivary neurons affect this modulation of the olivary burst size. Based on these results, we suggest that the olivary burst size might reflect the "expectancy" of a spike to occur rather than the spike timing, and that this process requires the presence of gap junction coupling.
Keywords: cerebellum; climbing fiber; gap junctions; inferior olive; subthreshold oscillations; wavelets.
Figures

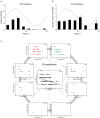

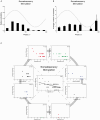
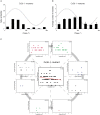


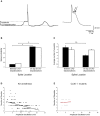
References
-
- Choi S., Yu E., Kim D., Urbano F. J., Makarenko V., Shin H. S., et al. (2010). Subthreshold membrane potential oscillations in inferior olive neurons are dynamically regulated by P/Q- and T-type calcium channels: a study in mutant mice. J. Physiol. 588, 3031–3043 10.1113/jphysiol.2009.184705 - DOI - PMC - PubMed
-
- Crill W. E. (1970). Unitary multiple-spiked responses in cat inferior olive nucleus. J. Neurophysiol. 33, 199–209 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

