Impact of genetic polymorphisms on chemotherapy toxicity in childhood acute lymphoblastic leukemia
- PMID: 23189085
- PMCID: PMC3504364
- DOI: 10.3389/fgene.2012.00249
Impact of genetic polymorphisms on chemotherapy toxicity in childhood acute lymphoblastic leukemia
Abstract
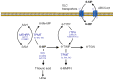
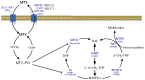
The efficacy of chemotherapy in pediatric acute lymphoblastic leukemia (ALL) patients has significantly increased in the last 20 years; as a result, the focus of research is slowly shifting from trying to increase survival rates to reduce chemotherapy-related toxicity. At the present time, the cornerstone of therapy for ALL is still formed by a reduced number of drugs with a highly toxic profile. In recent years, a number of genetic polymorphisms have been identified that can play a significant role in modifying the pharmacokinetics and pharmacodynamics of these drugs. The best example is that of the TPMT gene, whose genotyping is being incorporated to clinical practice in order to individualize doses of mercaptopurine. However, there are additional genes that are relevant for the metabolism, activity, and/or transport of other chemotherapy drugs that are widely use in ALL, such as methotrexate, cyclophosphamide, vincristine, L-asparaginase, etoposide, cytarabine, or cytotoxic antibiotics. These genes can also be affected by genetic alterations that could therefore have clinical consequences. In this review we will discuss recent data on this field, with special focus on those polymorphisms that could be used in clinical practice to tailor chemotherapy for ALL in order to reduce the occurrence of serious adverse effects.
Keywords: acute lymphoblastic leukemia; chemotherapy; genetic polymorphisms; pharmacogenetics; toxicity.
Figures
Similar articles
-
Genetic polymorphism of inosine-triphosphate-pyrophosphatase influences mercaptopurine metabolism and toxicity during treatment of acute lymphoblastic leukemia individualized for thiopurine-S-methyl-transferase status.Expert Opin Drug Saf. 2010 Jan;9(1):23-37. doi: 10.1517/14740330903426151. Expert Opin Drug Saf. 2010. PMID: 20021291 Review.
-
TPMT polymorphisms and minimal residual disease after 6-mercaptopurine post-remission consolidation therapy of childhood acute lymphoblastic leukaemia.Pediatr Hematol Oncol. 2021 Apr;38(3):227-238. doi: 10.1080/08880018.2020.1842570. Epub 2020 Nov 18. Pediatr Hematol Oncol. 2021. PMID: 33205673 Clinical Trial.
-
The Promise of Pharmacogenomics in Reducing Toxicity During Acute Lymphoblastic Leukemia Maintenance Treatment.Genomics Proteomics Bioinformatics. 2017 Apr;15(2):82-93. doi: 10.1016/j.gpb.2016.11.003. Epub 2017 Apr 6. Genomics Proteomics Bioinformatics. 2017. PMID: 28391009 Free PMC article. Review.
-
Impact of single nucleotide polymorphisms of cytarabine metabolic genes on drug toxicity in childhood acute lymphoblastic leukemia.Pediatr Blood Cancer. 2015 Apr;62(4):622-8. doi: 10.1002/pbc.25379. Epub 2015 Jan 3. Pediatr Blood Cancer. 2015. PMID: 25557962
-
Multilocus genotypes of relevance for drug metabolizing enzymes and therapy with thiopurines in patients with acute lymphoblastic leukemia.Front Genet. 2013 Jan 7;3:309. doi: 10.3389/fgene.2012.00309. eCollection 2012. Front Genet. 2013. PMID: 23335936 Free PMC article.
Cited by
-
Pharmacogenetics of asparaginase in acute lymphoblastic leukemia.Cancer Drug Resist. 2019 Jun 19;2(2):242-255. doi: 10.20517/cdr.2018.24. eCollection 2019. Cancer Drug Resist. 2019. PMID: 35582721 Free PMC article. Review.
-
Population Pharmacokinetics of High-Dose Methotrexate in Chinese Pediatric Patients With Acute Lymphoblastic Leukemia.Front Pharmacol. 2021 Jul 13;12:701452. doi: 10.3389/fphar.2021.701452. eCollection 2021. Front Pharmacol. 2021. PMID: 34326772 Free PMC article.
-
Potential neurotoxicity associated with methotrexate.Sci Rep. 2024 Aug 9;14(1):18548. doi: 10.1038/s41598-024-69263-0. Sci Rep. 2024. PMID: 39122917 Free PMC article.
-
Pharmacogenetics of pediatric acute lymphoblastic leukemia in Uruguay: adverse events related to induction phase drugs.Front Pharmacol. 2023 Nov 17;14:1278769. doi: 10.3389/fphar.2023.1278769. eCollection 2023. Front Pharmacol. 2023. PMID: 38044950 Free PMC article.
-
A single day of 5-azacytidine exposure during development induces neurodegeneration in neonatal mice and neurobehavioral deficits in adult mice.Physiol Behav. 2016 Dec 1;167:16-27. doi: 10.1016/j.physbeh.2016.08.036. Epub 2016 Sep 1. Physiol Behav. 2016. PMID: 27594097 Free PMC article.
References
-
- Adam de Beaumais T., Jacqz-Aigrain E. (2012). Pharmacogenetic determinants of mercaptopurine disposition in children with acute lymphoblastic leukemia. Eur. J. Clin. Pharmacol. 68 1233–1242 - PubMed
-
- Agundez J. A. (2004). Cytochrome P450 gene polymorphism and cancer. Curr. Drug Metab. 5 211–224 - PubMed
-
- Allan D. S., Kovacs M. J., Clark W. F. (2001). Frequently relapsing thrombotic thrombocytopenic purpura treated with cytotoxic immunosuppressive therapy. Haematologica 86 844–850 - PubMed
-
- Ban H., Andoh A., Imaeda H., Kobori A., Bamba S., Tsujikawa T., et al. (2010). The multidrug-resistance protein 4 polymorphism is a new factor accounting for thiopurine sensitivity in Japanese patients with inflammatory bowel disease. J. Gastroenterol. 45 1014–1021 - PubMed
LinkOut - more resources
Full Text Sources

