The role of water in activation mechanism of human N-formyl peptide receptor 1 (FPR1) based on molecular dynamics simulations
- PMID: 23189124
- PMCID: PMC3506623
- DOI: 10.1371/journal.pone.0047114
The role of water in activation mechanism of human N-formyl peptide receptor 1 (FPR1) based on molecular dynamics simulations
Abstract
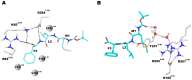
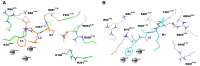
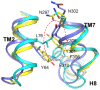
The Formyl Peptide Receptor 1 (FPR1) is an important chemotaxis receptor involved in various aspects of host defense and inflammatory processes. We constructed a model of FPR1 using as a novel template the chemokine receptor CXCR4 from the same branch of the phylogenetic tree of G-protein-coupled receptors. The previously employed template of rhodopsin contained a bulge at the extracellular part of TM2 which directly influenced binding of ligands. We also conducted molecular dynamics (MD) simulations of FPR1 in the apo form as well as in a form complexed with the agonist fMLF and the antagonist tBocMLF in the model membrane. During all MD simulation of the fMLF-FPR1 complex a water molecule transiently bridged the hydrogen bond between W254(6.48) and N108(3.35) in the middle of the receptor. We also observed a change in the cytoplasmic part of FPR1 of a rotamer of the Y301(7.53) residue (tyrosine rotamer switch). This effect facilitated movement of more water molecules toward the receptor center. Such rotamer of Y301(7.53) was not observed in any crystal structures of GPCRs which can suggest that this state is temporarily formed to pass the water molecules during the activation process. The presence of a distance between agonist and residues R201(5.38) and R205(5.42) on helix TM5 may suggest that the activation of FPR1 is similar to the activation of β-adrenergic receptors since their agonists are separated from serine residues on helix TM5. The removal of water molecules bridging these interactions in FPR1 can result in shrinking of the binding site during activation similarly to the shrinking observed in β-ARs. The number of GPCR crystal structures with agonists is still scarce so the designing of new ligands with agonistic properties is hampered, therefore homology modeling and docking can provide suitable models. Additionally, the MD simulations can be beneficial to outline the mechanisms of receptor activation and the agonist/antagonist sensing.
Conflict of interest statement
Figures








Similar articles
-
The Formyl Peptide Receptors: Diversity of Ligands and Mechanism for Recognition.Molecules. 2017 Mar 13;22(3):455. doi: 10.3390/molecules22030455. Molecules. 2017. PMID: 28335409 Free PMC article. Review.
-
Molecular docking of 2-(benzimidazol-2-ylthio)-N-phenylacetamide-derived small-molecule agonists of human formyl peptide receptor 1.J Mol Model. 2012 Jun;18(6):2831-43. doi: 10.1007/s00894-011-1307-x. Epub 2011 Nov 30. J Mol Model. 2012. PMID: 22127612 Free PMC article.
-
Structural determinants for the interaction of formyl peptide receptor 2 with peptide ligands.J Biol Chem. 2014 Jan 24;289(4):2295-306. doi: 10.1074/jbc.M113.509216. Epub 2013 Nov 27. J Biol Chem. 2014. PMID: 24285541 Free PMC article.
-
Structural basis of ligand binding modes at the human formyl peptide receptor 2.Nat Commun. 2020 Mar 5;11(1):1208. doi: 10.1038/s41467-020-15009-1. Nat Commun. 2020. PMID: 32139677 Free PMC article.
-
FPR1: A critical gatekeeper of the heart and brain.Pharmacol Res. 2024 Apr;202:107125. doi: 10.1016/j.phrs.2024.107125. Epub 2024 Mar 2. Pharmacol Res. 2024. PMID: 38438091 Review.
Cited by
-
Lipid receptor S1P₁ activation scheme concluded from microsecond all-atom molecular dynamics simulations.PLoS Comput Biol. 2013;9(10):e1003261. doi: 10.1371/journal.pcbi.1003261. Epub 2013 Oct 3. PLoS Comput Biol. 2013. PMID: 24098103 Free PMC article.
-
Recent Advances and Applications of Molecular Docking to G Protein-Coupled Receptors.Molecules. 2017 Feb 22;22(2):340. doi: 10.3390/molecules22020340. Molecules. 2017. PMID: 28241450 Free PMC article. Review.
-
In silico analysis reveals sequential interactions and protein conformational changes during the binding of chemokine CXCL-8 to its receptor CXCR1.PLoS One. 2014 Apr 4;9(4):e94178. doi: 10.1371/journal.pone.0094178. eCollection 2014. PLoS One. 2014. PMID: 24705928 Free PMC article.
-
The Formyl Peptide Receptors: Diversity of Ligands and Mechanism for Recognition.Molecules. 2017 Mar 13;22(3):455. doi: 10.3390/molecules22030455. Molecules. 2017. PMID: 28335409 Free PMC article. Review.
-
Development of small molecule non-peptide formyl peptide receptor (FPR) ligands and molecular modeling of their recognition.Curr Med Chem. 2014;21(13):1478-504. doi: 10.2174/0929867321666131218095521. Curr Med Chem. 2014. PMID: 24350845 Free PMC article. Review.
References
-
- Filep JG, El Kebir D (2009) Neutrophil apoptosis: a target for enhancing the resolution of inflammation. J Cell Biochem 108: 1039–1046. - PubMed
-
- Brandenburg LO, Seyferth S, Wruck CJ, Koch T, Rosenstiel P, et al. (2009) Involvement of Phospholipase D 1 and 2 in the subcellular localization and activity of formyl-peptide-receptors in the human colonic cell line HT29. Mol Membr Biol 26: 371–383. - PubMed
-
- Zhang Y, Syed R, Uygar C, Pallos D, Gorry MC, et al. (2003) Evaluation of human leukocyte N-formylpeptide receptor (FPR1) SNPs in aggressive periodontitis patients. Genes Immun 4: 22–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

