Performance of LBSap vaccine after intradermal challenge with L. infantum and saliva of Lu. longipalpis: immunogenicity and parasitological evaluation
- PMID: 23189161
- PMCID: PMC3506642
- DOI: 10.1371/journal.pone.0049780
Performance of LBSap vaccine after intradermal challenge with L. infantum and saliva of Lu. longipalpis: immunogenicity and parasitological evaluation
Abstract
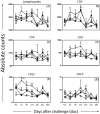
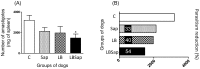
In the last decade, the search for new vaccines against canine visceral leishmaniasis has intensified. However, the pattern related to immune protection during long periods after experimental infection in vaccine trials is still not fully understood. Herein, we investigated the immunogenicity and parasitological levels after intradermal challenge with Leishmania infantum plus salivary gland extract in dogs immunized with a vaccine composed of L. braziliensis antigens plus saponin as an adjuvant (LBSap vaccine). The LBSap vaccine elicited higher levels of total anti-Leishmania IgG as well as both IgG1 and IgG2. Furthermore, dogs vaccinated had increased levels of lymphocytes, particularly circulating B cells (CD21(+)) and both CD4(+) and CD8(+) T lymphocytes. LBSap also elicited an intense in vitro cell proliferation associated with higher levels of CD4(+) T lymphocytes specific for vaccine soluble antigen and soluble lysate of L. infantum antigen even 885 days after experimental challenge. Furthermore, LBSap vaccinated dogs presented high IFN-γ and low IL-10 and TGF-β1 expression in spleen with significant reduction of parasite load in this tissue. Overall, our results validate the potential of LBSap vaccine to protect against L. infantum experimental infection and strongly support further evaluation of efficiency of LBSap against CVL in natural infection conditions.
Conflict of interest statement
Figures



References
-
- Desjeux P (2004) Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis 27: 305–318. - PubMed
-
- Dantas-Torres F (2007) The role of dogs as reservoirs of Leishmania parasites, with emphasis on Leishmania (Leishmania) infantum and Leishmania (Viannia) braziliensis. Vet Parasitol 149: 139–146. - PubMed
-
- WHO (2010) Control of the leishmaniasis. World Health Organ Tech Rep Ser. 1–186. - PubMed
-
- Courtenay O, Quinnell RJ, Garcez LM, Shaw JJ, Dye C (2002) Infectiousness in a cohort of brazilian dogs: why culling fails to control visceral leishmaniasis in areas of high transmission. J Infect Dis 186: 1314–1320. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

