Burn injury triggered dysfunction in dendritic cell response to TLR9 activation and resulted in skewed T cell functions
- PMID: 23189191
- PMCID: PMC3506591
- DOI: 10.1371/journal.pone.0050238
Burn injury triggered dysfunction in dendritic cell response to TLR9 activation and resulted in skewed T cell functions
Abstract
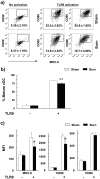
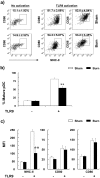
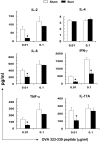
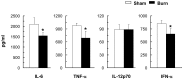
Severe trauma such as burn injury is often associated with a systemic inflammatory syndrome characterized by a hyperactive innate immune response and suppressed adaptive immune function. Dendritic cells (DCs), which sense pathogens via their Toll-like receptors (TLRs), play a pivotal role in protecting the host against infections. The effect of burn injury on TLR-mediated DC function is a debated topic and the mechanism controlling the purported immunosuppressive response remains to be elucidated. Here we examined the effects of burn injury on splenic conventional DC (cDC) and plasmacytoid DC (pDC) responses to TLR9 activation. We demonstrate that, following burn trauma, splenic cDCs' cytokine production profile in response to TLR9 activation became anti-inflammatory dominant, with high production of IL-10 (>50% increase) and low production of IL-6, TNF-α and IL-12p70 (∼25-60% reduction). CD4+ T cells activated by these cDCs were defective in producing Th1 and Th17 cytokines. Furthermore, burn injury had a more accentuated effect on pDCs than on cDCs. Following TLR9 activation, pDCs displayed an immature phenotype with an impaired ability to secrete pro-inflammatory cytokines (IFN-α, IL-6 and TNF-α) and to activate T cell proliferation. Moreover, cDCs and pDCs from burn-injured mice had low transcript levels of TLR9 and several key molecules of the TLR signaling pathway. Although hyperactive innate immune response has been associated with severe injury, our data show to the contrary that DCs, as a key player in the innate immune system, had impaired TLR9 reactivity, an anti-inflammatory phenotype, and a dysfunctional T cell-priming ability. We conclude that burn injury induced impairments in DC immunobiology resulting in suppression of adaptive immune response. Targeted DC immunotherapies to promote their ability in triggering T cell immunity may represent a strategy to improve immune defenses against infection following burn injury.
Conflict of interest statement
Figures





Similar articles
-
Murine dendritic cell antigen-presenting cell function is not altered by burn injury.J Leukoc Biol. 2009 May;85(5):862-70. doi: 10.1189/jlb.0408257. Epub 2009 Feb 19. J Leukoc Biol. 2009. PMID: 19228816 Free PMC article.
-
The involvement of endoplasmic reticulum stress response in immune dysfunction of dendritic cells after severe thermal injury in mice.Oncotarget. 2017 Feb 7;8(6):9035-9052. doi: 10.18632/oncotarget.14764. Oncotarget. 2017. PMID: 28118617 Free PMC article.
-
Human Plasmacytoid and Monocyte-Derived Dendritic Cells Display Distinct Metabolic Profile Upon RIG-I Activation.Front Immunol. 2018 Dec 21;9:3070. doi: 10.3389/fimmu.2018.03070. eCollection 2018. Front Immunol. 2018. PMID: 30622542 Free PMC article.
-
Inflammatory signals in dendritic cell activation and the induction of adaptive immunity.Immunol Rev. 2009 Jan;227(1):234-47. doi: 10.1111/j.1600-065X.2008.00718.x. Immunol Rev. 2009. PMID: 19120488 Review.
-
Regulation of dendritic cell function through Toll-like receptors.Curr Mol Med. 2003 Jun;3(4):373-85. doi: 10.2174/1566524033479726. Curr Mol Med. 2003. PMID: 12776992 Review.
Cited by
-
Sex-related immunity: could Toll-like receptors be the answer in acute inflammatory response?Front Immunol. 2024 May 21;15:1379754. doi: 10.3389/fimmu.2024.1379754. eCollection 2024. Front Immunol. 2024. PMID: 38835761 Free PMC article. Review.
-
IL-15 Superagonist Expands mCD8+ T, NK and NKT Cells after Burn Injury but Fails to Improve Outcome during Burn Wound Infection.PLoS One. 2016 Feb 9;11(2):e0148452. doi: 10.1371/journal.pone.0148452. eCollection 2016. PLoS One. 2016. PMID: 26859674 Free PMC article.
-
The Role of CHI3L1 (Chitinase-3-Like-1) in the Pathogenesis of Infections in Burns in a Mouse Model.PLoS One. 2015 Nov 3;10(11):e0140440. doi: 10.1371/journal.pone.0140440. eCollection 2015. PLoS One. 2015. PMID: 26528713 Free PMC article.
-
Frontline Science: Anti-PD-L1 protects against infection with common bacterial pathogens after burn injury.J Leukoc Biol. 2018 Jan;103(1):23-33. doi: 10.1002/JLB.5HI0917-360R. Epub 2017 Dec 21. J Leukoc Biol. 2018. PMID: 29345058 Free PMC article.
-
Perturbed MafB/GATA1 axis after burn trauma bares the potential mechanism for immune suppression and anemia of critical illness.J Leukoc Biol. 2016 Oct;100(4):725-736. doi: 10.1189/jlb.1A0815-377R. Epub 2016 Mar 18. J Leukoc Biol. 2016. PMID: 26992433 Free PMC article.
References
-
- Hoover L, Bochicchio GV, Napolitano LM, Joshi M, Bochicchio K, et al.. (2006) Systemic inflammatory response syndrome and nosocomial infection in trauma. J Trauma 61: 310–316; discussion 316–317. - PubMed
-
- Sauaia A, Moore FA, Moore EE, Lezotte DC (1996) Early risk factors for postinjury multiple organ failure. World J Surg 20: 392–400. - PubMed
-
- Rodeberg DA, Meyer JG, Babcock GF (1999) Heat shock response: presence and effects in burn patient neutrophils. J Leukoc Biol 66: 773–780. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

