Developmental origin of a major difference in sensory patterning between zebrafish and bluefin tuna
- PMID: 23189756
- PMCID: PMC3488297
- DOI: 10.1111/j.1525-142x.2012.00529.x
Developmental origin of a major difference in sensory patterning between zebrafish and bluefin tuna
Abstract
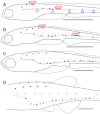
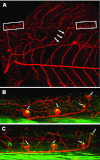
The posterior lateral line system (PLL) of teleost fish comprises a number of mechanosensory organs arranged in defined patterns on the body surface. Embryonic patterns are largely conserved among teleosts, yet adult patterns are highly diverse. Although changes in pattern modify the perceptual abilities of the system, their developmental origin remains unknown. Here we compare the processes that underlie the formation of the juvenile PLL pattern in Thunnus thynnus, the bluefin tuna, to the processes that were elucidated in Danio rerio, the zebrafish. In both cases, the embryonic PLL comprises five neuromasts regularly spaced along the horizontal myoseptum, but the juvenile PLL comprises four roughly parallel anteroposterior lines in zebrafish, whereas it is a simple dorsally arched line in tuna fish. We examined whether this difference involves evolutionary novelties, and show that the same mechanisms mediate the transition from embryonic to juvenile patterns in both species. We conclude that the marked difference in juveniles depends on a single change (dorsal vs. ventral migration of neuromasts) in the first days of larval life.
Figures




References
-
- Aman A, Piotrowski T. Cell migration during morphogenesis. Dev. Biol. 2010;341:20–33. - PubMed
-
- Coombs S, Montgomery JC. The enigmatic lateral line system. In: Fay RR, Popper AN, editors. Comparative Hearing: Fish and Amphibians. New York: Springer Verlag; 1999. pp. 319–362.
-
- Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2009;10:445–457. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

