Depot medroxyprogesterone acetate increases immune cell numbers and activation markers in human vaginal mucosal tissues
- PMID: 23189932
- PMCID: PMC3581024
- DOI: 10.1089/aid.2012.0271
Depot medroxyprogesterone acetate increases immune cell numbers and activation markers in human vaginal mucosal tissues
Abstract
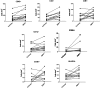
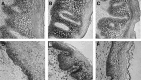
The relationship between exogenous contraceptive hormones and permissiveness of the female genital tract to human immunodeficiency virus type 1 (HIV-1) is the subject of renewed debate. To better characterize the effect of depot medroxyprogesterone acetate (DMPA) on HIV-1 cellular targets and epithelial integrity in the vagina, we compared leukocyte populations, markers of activation and proliferation, and the density of intercellular junctional proteins in the vaginal epithelium of women during the follicular and luteal phases of the menstrual cycle and approximately 12 weeks after receiving a DMPA injection. This prospective cohort study involved 15 healthy women. Vaginal biopsies were obtained in the follicular and luteal phases of the menstrual cycle, and approximately 12 weeks following a 150-mg intramuscular injection of DMPA. Leukocyte populations, activation phenotype, and epithelial tight junction and adherens proteins were evaluated by immunohistochemistry. After receiving DMPA, the numbers of CD45, CD3, CD8, CD68, HLA-DR, and CCR5 bearing immune cells were significantly (p<0.05) increased in vaginal tissues, compared to the follicular and/or luteal phases of untreated cycles. There were no significant differences in immune cell populations between the follicular and luteal phases of the control cycle. There were also no statistically significant differences in epithelial thickness and density of epithelial tight junction and adherens proteins among the follicular, luteal, and post-DMPA treatment sampling points. In this pilot study, vaginal immune cell populations were significantly altered by exogenous progesterone, resulting in increased numbers of T cells, macrophages, and HLA-DR- and CCR5-positive cells.
Figures

References
-
- UNAIDS. World AIDS Day Report. 2011.
-
- Kaunitz AM. Long-acting injectable contraception with depot medroxyprogesterone acetate. Am J Obstet Gynecol. 1994;170(5 Pt 2):1543–1549. - PubMed
-
- WHO. Medical Eligibility Criteria for Contraceptive Use 2008 Update. 2008.
-
- Mauck CK. Callahan MM. Baker J, et al. The effect of one injection of Depo-Provera on the human vaginal epithelium and cervical ectopy. Contraception. 1999;60(1):15–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

